In Situ Chemical Oxidation
IntroductionIn situ chemical oxidation (ISCO) is the most rapidly growing remedial technology applied at EPA hazardous waste sites. ISCO involves the introduction of a chemical oxidant into the subsurface for the purpose of transforming groundwater or soil contaminants into less harmful chemical species. ISCO results in the transformation of a wide range of environmental contaminants, enhances mass transfer, and is being used at both old sites and newly discovered hazardous waste sites. ISCO is specifically used to reduce contaminant mass and concentrations in soil and groundwater, contaminant mass flux from source areas to downgradient pump-and-treat systems, and to reduce anticipated cleanup times required for natural attenuation and other remedial options. There are several different forms of oxidants that have been used for ISCO; however, the most commonly used oxidants include:
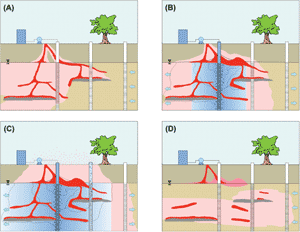
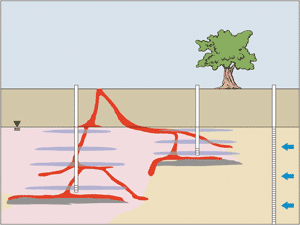
The two most commonly used forms of injected oxidants are permanganate and Fenton’s. (Refer to Figure 1.) The persistence of the oxidant in the subsurface is important since this affects the contact time for advective and diffusive transport, and ultimately the extent to which the oxidant can be delivered to subsurface targeted zones. For example, permanganate persists for long periods of time and, therefore, both diffusion into low-permeability materials and greater transport (advective) distances through porous media are possible. H2O2 has been reported to persist in soil and aquifer material for minutes to hours and the relative diffusive and advective transport distances will be limited. Efforts to stabilize the reaction rate in the subsurface are needed to enhance transport distances and persistence. Radical intermediates formed using H2O2, S2O82-, and O3 are generally considered to be responsible for contaminant transformations. These intermediates react very quickly and persist for very short periods of time (less than 1 second). (Refer to Figure 2.) Permanganate-based ISCO is more fully developed than the other forms of oxidant. Widespread use of in situ permanganate oxidation (involving a diversity of contaminants and geological environments under well documented pilot- and field-scale conditions) in conjunction with long term monitoring data and cost information has contributed to the development of the technology and the infrastructure needed to support decisions to deploy and design permanganate ISCO systems. However, additional research and development is still needed. Fenton oxidation involves the injection of H2O2 alone or in combination with other coinjected chemical reagents, including various forms of iron (Fe+2), acid/base, and chelating agents. The main Fenton reactions have been previously investigated; however, side and competing reactions that may play major roles in contaminant transformations have not been fully investigated. Additional research is required. Complex heterogeneous systems involving aquifer materials, soils, and groundwater introduce potential treatment inefficiencies due to imperfect reactive conditions. Consequently, EPA’s scientific investigations are focused on both fundamental and applied aspects of chemical oxidation. In this way, we can identify and understand site conditions and treatment process variables that contribute to effective and efficient treatment methods. Fenton-driven ISCO has been deployed at a large number of sites and involves a variety of approaches and methods. In general, Fenton chemistry and in situ Fenton oxidation are complex. They involve numerous reactive intermediates and mechanisms and the technology has been slower to develop. Ozone is a strong oxidant that has been used in the subsurface, but in limited application relative to permanganate, Fenton-driven, and persulfate oxidation. Persulfate (S2O82-) is a relatively new form of oxidant that has mainly been investigated at bench-scale. However, considerable research is being conducted on this oxidant and its applied use at an increasing number of laboratories and field sites, resulting in the rapid development of this technology. ContactDr. Scott G. Huling Dr. David Burden Adsorption/OxidationA Fenton-driven mechanism for regenerating spent granular activated carbon (GAC) involves the combined, synergistic use of two treatment technologies: adsorption on activated carbon and Fenton oxidation. During carbon adsorption treatment, environmental contaminants are immobilized and concentrated on the GAC, and subsequently transformed by hydroxide (OH) (a strong, nonspecific oxidant) or other reaction intermediates. The objective of the treatment process is to:
Activated carbon is the most commonly used adsorbent in the United States today. The U.S. consumption of activated carbon was 400 million pounds in 2001. This mass of activated carbon is growing each year and the international market even greater. Activated carbon is used as a broad-spectrum adsorbent to purify air, water, and wastewater under numerous environmental programs implemented via EPA (e.g., Clean Water Act, Clean Air Act, Safe Drinking Water Act, Resource Conservation and Recovery Act, underground storage tank regulations, and Comprehensive Environmental Response, Compensation, and Liability Act [Superfund]). Similarly, a wide range of environmental contaminants react with OH, with moderately high reaction-rate constants. Therefore, a wide range of contaminant classes are amenable to treatment via the adsorption/oxidation process. The technology could be used on-site and in situ in either above- or below-ground hydraulic configurations, providing an efficient means by which to destroy a wide range of toxic compounds. This treatment process yields highquality treatment effluent and can be deployed under a variety of applications. Preliminary results indicate low costs and favorable economics and feasibility, suggesting this is a viable alternative to conventional thermal regeneration where costs can be high due to transportation, treatment, air pollution control, and process inefficiencies. A preliminary analysis indicates that the technology is green relative to thermal regeneration methods and would likely result in a reduction in the consumption of fossil fuels and formation of greenhouse gases. (Refer to Figure 3.) See AlsoGround Water Technical Support Center References and ProductsSelected Publications Huling, S.G., E. Kan, C. Wingo, and S. Park. (2011a). (Accepted). “Pilot-Scale Demonstration of Fenton-Driven Regeneration of MTBE-Spent Granular Activated Carbon.” J. Haz. Mat. Ko, S., S.G. Huling, and B. Pivetz. (2011). (Accepted). “Ground Water Sample Preservation at In Situ Chemical Oxidation Sites – Recommended Guidelines.” EPA Ground Water Forum Issue Paper. U.S. Environmental Protection Agency, National Risk Management Research Laboratory, R.S. Kerr Environmental Research Center, Ada, OK. (EPA/600/R-11/109) Huling, S.G., S. Hwang, D. Fine, and S. Ko. (2011). “Fenton-Like Initiation of a Toluene Transformation Mechanism.” Wat. Res., 45: 5334–5342. Huling, S.G., S. Ko, and B. Pivetz. (2011). “Ground Water Sampling at ISCO Sites – Binary Mixtures of Volatile Organic Compounds and Persulfate.” Ground Water Monit. Remed., 31, 2: Spring 72–79. Huling, S.G., S. Ko, S. Park, and E. Kan. (2011). “Persulfate-Driven Oxidation of Contaminant-Spent Granular Activated Carbon.” J. Haz. Mat., 192, 9(3: 1484–1490. Huling, S. and S. Hwang. (2010). “Iron Amendment and Fenton Oxidation of MTBE-Spent Granular Activated Carbon.” Wat. Res., 44, 8: 2663–2671. Hwang, S., S.G. Huling, and S. Ko. (2010). “Fenton-Like Degradation of MTBE: Effects of Iron Counter Anion and Radical Scavengers.” Chemosphere, 78, 5: 563–568. Huling, S.G., E. Kan, and C. Wingo. (2009). “Fenton-Driven Regeneration of MTBE-Spent Granular Activated Carbon – Effects of Particle Size and Iron Amendment Procedures”. J. Applied Catalysis B: Environmental, 89: 651-657. Kan, E. and S.G. Huling. (2009). “Effects of Temperature and Acidic Pre-Treatment on Fenton-Driven Oxidation of MTBE-Spent Granular Activated Carbon.” Environmental Science and Technology, 43, 5: 1493–1499. Huling, S.G., K.P Jones, and T. Lee. (2007). “Iron Optimization for Fenton-Driven Oxidation of MTBE-Spent Granular Activated Carbon.” Environmental Science and Technology, 41, 11: 4090–4096. In Situ Regeneration of Granular Activated Carbon (GAC) Using Fenton’s Reagents, Final Prpoject Report (EPA/600/R-07/008) November 2007 | Abstract De Las Casas, C., K. Bishop, L. Bercik, M. Johnson, M. Potzler, W. Ela, A.E. Sáez, S. Huling, and R. Arnold. (2006). “In-Place Regeneration of GAC Using Fenton’s Reagents.” In: Proceedings Innovative Approaches for the Remediation of Subsurface-Contaminated Hazardous Waste Sites: Bridging Flask and Field Scales, ACS Symposium Series 940, 43–65. In Situ Chemical Oxidation – Engineering Issue (EPA/600/R-06/072) August 2006 Huling, S.G., P.K. Jones, W.P. Ela, and R.G. Arnold. (2005). “Fenton-Driven Chemical Regeneration of MTBE-Spent Granular Activated Carbon.” Water Research, 39: 2145–2153. Huling, S.G., P.K. Jones, W.P. Ela, and R.G. Arnold. (2005). “Repeated Reductive and Oxidative Treatments on Granular Activated Carbon.” Journal of Environmental Engineering, 131, 2: 287–297. Lin, Z. and S.G. Huling. (2005). “Determination of Chlorophenols, Nitrophenols, and Methylphenols in Ground Water Using High-Performance Liquid Chromatography.” Hydrol. Sci. and Technol. J., 20, 1-4: 101–110. Kommineni, S., W.P. Ela, R.G. Arnold, S.G. Huling, B.J. Hester, and E.A. Betterton. (2003). “NDMA Treatment by Sequential GAC Adsorption and Fenton-Driven Destruction.” J. Environ. Eng. Sci., 20, 4: 361–373. Huling, S.G., B. Pivetz, and R. Stransky. (2002). “Terminal Electron Acceptor Mass Balance: NAPLs and Natural Attenuation.” Journal of Environmental Engineering, 128, 3: 246–252. Huling, S.G., R.G. Arnold, R.A. Sierka, and M.A. Miller. (2001). “Influence of Peat on Fenton Oxidation.” Water Research, 35, 7: 1687–1694. Huling, S.G., R.G. Arnold, P.K. Jones, and R.A. Sierka. (2000). “Predicting the Rate of Fenton-Driven 2-Chlorophenol Transformation Using a Contaminant Analog.” Journal of Environmental Engineering, 126, 4: 348–353. Huling, S.G., R.G. Arnold, R.A. Sierka, P.K. Jones, and D. Fine. (2000). “Contaminant Adsorption and Oxidation via Fenton Reaction.” Journal of Environmental Engineering, 126, 7: 595–600. McCaulou, D.R. and S.G. Huling. (1999). “Compatibility of Bentonite and DNAPLs.” Journal of Ground Water Monitoring and Remediation, 19, 2: 78–86. Huling, S.G., R.G. Arnold, R.A. Sierka, and M.A. Miller. (1998). “Measurement of Hydroxyl Radical Activity in a Soil Slurry Using the Spin Trap a-(4 pyridyl-1-oxide)-N-tert-butylnitrone.” Environmental Science and Technology, 32, 21: 3436–3441. Patents Huling, S.G., R.G. Arnold, and R.A. Sierka. (2008). “Contaminant Adsorption and Oxidation via Fenton Reaction.” Patent US 7,335,246. Huling, S.G., R.G. Arnold, and R.A. Sierka. (2003). “Contaminant Adsorption and Oxidation via Fenton Reaction.” Patent US 6,663,781, filed August 28, 2000. ContactDr. Scott G. Huling
You will need Adobe Reader to view some of the files on this page. |
![[logo] US EPA](../gif/logo_epaseal.gif)