T-REX Version 1.4.1(SUPERSEDED) User's Guide for Calculating Pesticide Residues on Avian and Mammalian Food Items
December 11, 2008
Environmental Fate and Effects Division
Office of Pesticide Programs
U.S. Environmental Protection Agency
Washington, D.C.
On this page
- Introduction
- Guidance on T-REX Inputs
- T-REX Calculations and Results
- Risk Description Points to Consider
| T-REX User's Guide and Spreadsheet tool |
Contributors | QA/QC Officers | QA/QC Testers | QA/QC Reviewers |
|---|---|---|---|---|
|
|
|
|
|
The authors would also like to thank the members of the Terrestrial Biology and Terrestrial Exposure Technology Teams for their input and assistance in the development of this User's Guide and Spreadsheet.
-
Introduction
Welcome to the Terrestrial Residue EXposure (T-REX) spreadsheet tool User's Guide. This document will provide directions on how to use T-REX and also an explanation of how exposure concentrations and risk quotients are estimated.
This spreadsheet-based model calculates the residues on avian and mammalian food items along with the dissipation rate of a chemical applied to foliar surfaces (for single or multiple applications) in order to estimate acute and reproductive risk quotients. LD50 ft-2 values are calculated for both broadcast and banded (granular and liquid) applications using an adjusted LD50 method. The results are presented by weight class for various sized birds and mammals for each type of application. Avian and mammalian risk quotients are also calculated for seed treatment applications to various crop seeds.
T-REX uses the same principle as the batch code models FATE and TERREEC, which calculate terrestrial exposure concentration estimates on plant surfaces following pesticide application. However, T-REX performs a number of calculations that neither FATE nor TERREEC perform. For example, T-REX adjusts acute and chronic toxicity values based on the relative body weight of the animal being assessed compared with the animal used in the toxicity studies. T-REX also calculates risk quotients for granular applications and seed treatments.
T-REX v.1.4.1 has undergone a formal internal Quality Control (QC) review in the Environmental Fate and Effects Division (EFED). A QC Plan was developed and then approved by the Project Team and the QC Manager, which was then implemented and then reviewed. All errors found were addressed and/or corrected by the QC reviewer and follow up QC checks were done.
When T-REX is opened, a warning message may indicate that "because security risk is high, the macros have been disabled." In this case, click on "Tools" -> "Macro" -> "Security" and change the security level from high to medium. Click "Enable Macros" whenever prompted.
Once T-REX is opened, the "READ ME" worksheet is displayed (Figure 1-1). This worksheet contains the version information, new model updates, and a list of references. Across the bottom of the Excel window are several worksheet tabs, indicating the various functions performed by T-REX.
The User's Guide provides guidance to the assessor on inputs needed to run T-REX (Section 2), describes the calculations performed by T-REX and the results (Section 3), and provides guidance on characterizing the results in the risk description of an ecological risk assessment (Section 4). For questions that are not explained in this manual, the user should contact the program authors, Brian Anderson (anderson.brian@epa.gov) or Edward Odenkirchen(odenkirchen.edward@epa.gov). This manual is intended to provide general guidance on the use of T-REX and is not intended to serve as a comprehensive guide on EFED's terrestrial risk assessment policies and methods.
Submitting Comments on T-REX, Version 1.4.1
The EFED user can submit comments on T-REX by clicking on the "questions, comments" button in the inputs and results worksheets. Clicking the comments button will open a new worksheet. To submit a comment, type your last name in Cell B3 and your comment in Cell B6. Exit out of cell B6, then click on the "submit comment" button. A comment will not be sent unless you exit out of cell B6. These comments will be used in creating future versions of the model; therefore, all users are strongly encouraged to submit comments. Users not in EFED or without access to the shared drive (F: drive) cannot submit comments.
 Figure 1-1
Figure 1-1
T-REX "Read Me" WorksheetT-REX has been designed to be easy to use, yet maintain a level of flexibility needed for the multitude of chemicals and use patterns encountered by risk assessors. Throughout the spreadsheet, look for small red cell tags that contain additional information and move the cursor over them to display the comment box. This User's Guide is organized into two general sections in addition to this Introduction (Section 1). Section 2 provides guidance on input parameters needed to run T-REX. Section 3 describes how calculations are made and provides guidance on how the results may be implemented in the risk assessment of pesticides. T-REX may be used for foliar applications, seed treatments, or LD50/ft2 analysis. Specific guidance for each analysis is included in this User's Guide.
-
1.1 Summary of New Version Updates
T-REX version 1.4.1 replaces T-REX version 1.3.1. A summary of the updates/changes that were made to v.1.3.1 are listed below; this list of updates can also be found on the "Read Me" page of the T-REX v.1.4.1 model spreadsheet.
-
T-REX was altered to allow the user to define the body weight range of assessed animals.
-
The background of dark colored cells was changed to white to enhance printing and scanning of documents.
-
Calculation of the LD50/square foot equation for liquids was altered and in version 1.4.1, it is based on the application rate in mass per unit area. In version 1.3.1, the calculation was performed only on the basis of volume per unit area with an assumption of density.
-
The calculation for the number of days levels of concern (LOCs) are exceeded was corrected and simplified.
-
Mammals were added to the calculation of the number of consumed granules that result in LOC exceedances; previous versions only included calculation of consumed granules for birds; and
-
Calculations were added for granivorous birds. Previous versions only included calculations for granivorous mammals.
-
-
-
Guidance on T-REX Inputs
This section of the document provides guidance for entering parameters into T-REX (v. 1.4.1) to calculate risk indices for use in ecological risk assessments. Inputs include application data (used to calculate estimated environmental concentrations, Section 2.1) and toxicity values (Section 2.2). Section 3 of this document describes how these input parameters are used by T-REX to calculate EECs and risk indices (risk quotients or LD50/ft2).
-
2.1. Entering Pesticide Application Data
An "Inputs" worksheet (Figure 2-1) has been included to increase consistency and transparency in the terrestrial exposure estimation process. With the exception of the seed treatment exposure and granular characterization worksheets, all necessary inputs may only be entered into the "Input" worksheet. Additional inputs needed for the granular characterization and seed treatment applications are described in Sections 2.4 and 3.2, respectively. All data should be entered into the blue cells only.
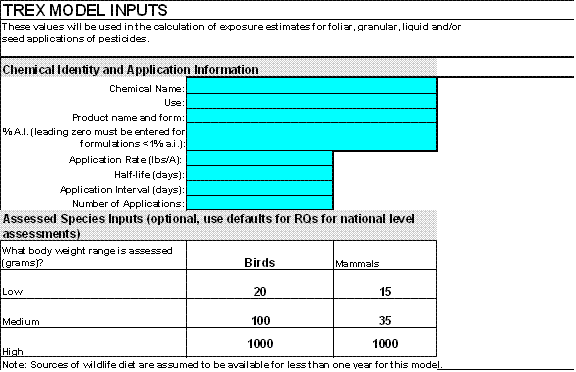 Figure 2-1
Figure 2-1
Inputs Screen in T-REX Version 1.4.1Inputs needed to run T-REX include the following (inputs denoted with an * are required; inputs denoted with a *C are required when multiple applications are being modeled:
Inputs to T-REX Input Name Input Description Chemical name Enter either the chemical or common name used in the assessment. Use Enter the type of use (e.g., turf, residential, crop, foliar, right-of-way, etc.) If applicable, also provide crop name Product name and form Enter the name of the formulated product as it appears on the subject label, including any indication of the form of the product (e.g., 10g — 10% granular, wp — wettable powder, etc.) % A.I.* If an application rate for a formulated product is used, enter the % a.i. for the formulation. However, if the application rate has already been adjusted for % a.i., use 100%.
If the percent a.i. is < 1%, then a leading zero to the left of the decimal must be entered to ensure that 0.5% is not changed to 50% by EXCEL. For example, a 0.5% formulation must be entered as "0.5" or ".5%" not ".5 ", and a 0.006% formulation must be entered as "0.006" or ".006%" not ".006".Application Rate* Enter the maximum label application rate (pounds a.i./acre). If your application rate has already been adjusted for active ingredient, make sure you have entered 100 in the % a.i. cell above; otherwise, enter your application rate in lbs formulation/A here and then the % a.i. in the product in the above cell. Foliar Dissipation Half-life* Enter the foliar dissipation half-life in days. EFED policy requires the risk assessor to consult the following HED studies to determine the pesticide residue half-life of wildlife food items used for terrestrial wildlife exposure modeling: available magnitude of residue (171-4), reduction of residue (171-5), and foliar dissipation (132-1).
Another source of dissipation data for wildlife exposure modeling purposes is Willis and McDowell (1987; F:\USER\SHARE\Models\TerrestrialExposure\TREX). In the event that the above data sources do not provide a suitable half-life estimate, the EFED default value is 35 days. When using the default, the risk assessor is urged to evaluate the effect of alternative assumptions of residue half-life on the outcome of the terrestrial wildlife risk assessment and include this evaluation in the discussion of risk assessment uncertainties.Application Interval*c Enter the minimum interval (days) between multiple applications (if any). Note: This version of the model can only accommodate uniform application intervals; subsequent models may incorporate a provision of intervals of varying length. The risk assessor may evaluate the effect of using application intervals other than the minimum interval on the label to explore the effects of possible mitigation options on risk outcomes. Number of Applications*c Enter the number of applications. EFED policy states that screening-risk assessments should consider the maximum number of applications specified on the product label. However, the risk assessor may elect other numbers of applications to explore the effects of possible mitigation efforts on risk outcomes. NOTE: "Clicking" the "reset model" button to the right of the first set of inputs will clear ALL of the user-supplied information. This button was included to allow the user to more quickly run multiple scenarios with T-REX without having to manually clear each cell.
-
2.2. Entering Toxicity Endpoint Data
To calculate risk quotients, user-supplied avian and mammalian toxicity endpoints need to be entered into the "Endpoints" section of the Inputs worksheet. These endpoints can be found in avian acute oral LD50, avian acute dietary LC50, avian reproduction NOAEC/L, acute mammalian LD 50 or LC50, and mammalian reproduction NOAEC/L toxicity studies.
T-REX requires that both the chosen endpoint (entered in the blue cell) and the test species be included (chosen from the drop-down menu options). For example, the user can enter an avian LD50 of 500 mg/kg-bw and this endpoint is based on a bobwhite quail study (i.e., chosen from the drop-down menu immediately to the right of the LD50 input cell). Typically, endpoint data for bobwhite quail, mallard duck, and laboratory rats from submitted studies or open literature will be used. In the case where data on bird species other than bobwhite quail or mallard duck are assessed (e.g., Japanese quail), choose "other" from the species drop down menu and enter the body weight of the species. A similar option for mammals is not currently available in this version of T-REX. Calculations for animals other than rats need to be done by hand using equations included in this User's Guide. Contact the program authors for assistance if needed, Brian Anderson (anderson.brian@epa.gov) or Edward Odenkirchen (odenkirchen.edward@epa.gov).
-
2.2.1. Avian Endpoints
The following avian toxicity endpoints may be entered into T-REX (Figure 2-2):
Avian Toxicity Endpoint Inputs Endpoint Name Endpoint Description LD50 Enter LD50 for dose-based acute effects endpoint (mg/kg-bw). LC50 Enter LC50 for dietary-based acute effects endpoint (mg/kg- diet). Reported Chronic Endpoint Enter NOAEL if reported in mg/kg-bw or NOAEC if reported in mg/kg-diet. Mineau Scaling Factor If chemical-specific data are available, the user can specify the avian scaling factor (see Mineau, et al.,1996) Otherwise, enter the EFED default value of (1.15). T-REX will not run unless a Mineau scaling factor is entered. However, zero can be entered if the assessor believes that body weight does not influence toxicity of the chemical being assessed. 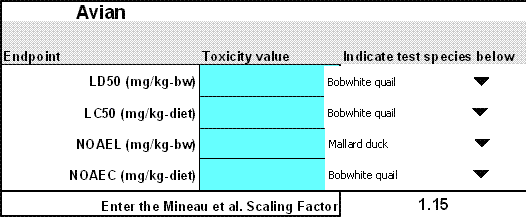 Figure 2-2
Figure 2-2
Avian Endpoints T-REX Version 1.4.1EFED policy states that for screening-level risk assessments, the lowest LD50, LC50, No Observable Adverse Effect Concentration (NOAEC) and/or No Observable Adverse Effect Level (NOAEL) from acceptable or supplemental studies should be used. For non-screening level risk assessments, the risk assessor may elect other values as necessary, but must document in the risk assessment why the lowest tested endpoint was not used. However, the assessor should determine that the lowest toxicity value from a laboratory study results in the lowest adjusted toxicity values for the various weight classes being assessed (See Section 3.1 for details on adjusted toxicity values). When toxicity values from different species are similar to each other, it is important to indicate because T-REX adjusts the toxicity values based on body weight and food intake of the tested organism compared with the assessed organism. Therefore, when data are available from multiple species, adjusted LD50s (described in Section 3) should be calculated for all species, and the lowest value should be used.
The risk assessor should note that this version of the model assumes a set body weight for tested bobwhite quail (178 g) and mallard duck (1580 g) unless another body weight is entered to the right of this cell or "other" is selected for a test species. Use of "other" species or studies involving subject animals markedly different from the assumed body weights requires the risk assessor to provide the alternate body weight and the species name. These data should be obtained from the study report if possible (time weighted average). Alternatively, referenced body weight values may be obtained from a variety of sources, including USEPA (1993) and the testing laboratory.
-
2.2.2. Mammalian Endpoints
The following mammalian endpoints may be entered into T-REX (Figure 2-3):
Mammalian Toxicity Endpoint Inputs Endpoint Name Endpoint Description LD50 Enter LD50 (mg/kg-bw) for dose-based acute effects endpoint. LC50 Enter LC50 (mg/kg-diet) for dietary-based acute effects endpoint. Reported Chronic Endpoint Enter NOAEL if reported in mg/kg-bw or NOAEC if reported in mg/kg-diet.
Enter a NOAEL and/or NOAEC. To the right of the cell is a drop down menu for the units in which the endpoint is expressed. The user may enter both a dietary concentration (NOAEC) and a daily dose (mg/kg-bw) if both are available. However, if both values are not available, then input "no" where T-REX asks if you have a dietary concentration or daily dose (T-REX will ask if you have the value not entered in Cell C30). If a dietary concentration and a daily dose are not both available, then the model will automatically make default adjustments for units to express the endpoint BOTH as NOAEC and NOAEL. The model will assume that a laboratory rat consumes 5% of its body weight daily (i.e., divide the concentration in food (ppm) by 20 to calculate the dose in mg/kg-bw). However, if a reproduction study reports toxicity values in units of dose (mg/kg-bw) and dietary concentration (mg/kg-diet), then both of these values should be used over the estimated values calculated by T-REX.
If a rat reproduction study is unavailable, the risk assessor may use the rat teratogenicity study NOAEL or NOAEC. In this case, the alternative endpoint should be identified and its limitations should be discussed in the risk description. Studies other than reproduction or developmental toxicity studies are not typically used by EFED to calculate risk quotients.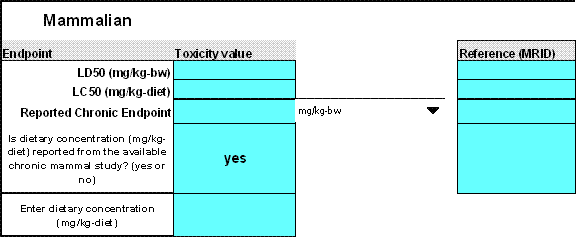 Figure 2-3
Figure 2-3
Mammalian Toxicity Endpoints T-REX Version 1.4.1The lowest LD50, LC50, NOAEC and/or NOAEL from acceptable or supplemental studies are used in screening-level risk assessments of mammals. For non-screening level assessments, the risk assessor may select other values as necessary, but must document in the risk assessment why the lowest tested endpoint was not used.
The risk assessor should note that this version of the model assumes a set body weight of 350 g for the tested organism, assuming they are laboratory rats. Use of other species or studies involving subject animals markedly different from the assumed body weight of 350 g will result in inaccuracies when extrapolating test endpoints to modeled animals. Therefore, if a mammalian species other than a rat is used (e.g., dog, rabbit, mink), the time-weighted average body weight of the animals that were tested in the experiment (from the DER or the original study report) should be used. Alternatively, reference body weight values may be obtained from a variety of sources including USEPA (1993) and the testing laboratory. However, T-REX does not currently allow the user to enter body weights of mammals that differ from the 350-gram laboratory rat. Therefore, adjusted toxicity values would need to be performed by hand using equations presented in this User's Guide.
-
-
2.3. Additional Inputs for LD50 ft-2 Analysis
T-REX includes the capability to calculate the LD50 ft-2 risk index values with the user supplied information (Figure 2-3 ). Conceptually, an LD50 ft-2 is the amount of a pesticide estimated to kill 50% of exposed animals in each square foot of applied area. Although a square foot does not have defined ecological relevance, and any unit area could be used, risk presumably increases as the number of LD50 s/ft2 increases. The LD50/ft2 is used to estimate risk for granular formulations and row, banded, and in-furrow applications. For additional information on the LD50 ft-2 risk index, please refer to USEPA (1992). The LD50 ft-2 is calculated using a toxicity value (adjusted LD50) and the EEC (mg a.i. ft-2) and is directly compared with the Agency's levels of concern (LOCs). Additional toxicity data are not needed for this calculation; however, additional information may be needed to allow for calculation of the exposure index (mg a.i./ft2 ). These data are described below.
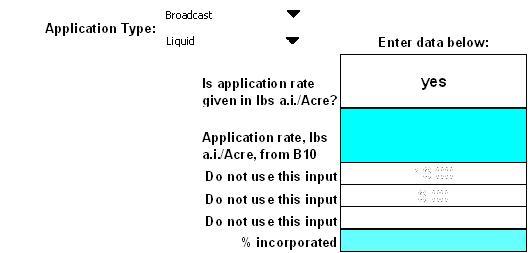 Figure 2-3
Figure 2-3
LD50 ft-2 inputsIn the first drop down menu, choose the method of application from either "Broadcast" or "Rows/Band/In-furrow." The method of application is important in LD50 ft-2 calculations as it is linked to assumptions regarding the availability of the pesticide to wildlife. In the second drop down menu, choose from either "Granular" or "Liquid". The application media is important for bioavailability assumptions for LD50 ft-2 calculations. Information regarding these inputs may be obtained from the product label.
Depending on the combination of application method chosen and characteristics of formulated product, the user will be prompted to enter the appropriate data in the blue cells located below and to the right of the drop down menus. For broadcast applications of granular products, the user does not need to enter any additional information on the "inputs" page. However, if the product is a liquid applied as a broadcast spray or is a liquid or granular and is applied in rows, then additional information is needed. Guidance for entering data for each type of application is provided in Table 2-1 below. The user should pay attention to the units being requested for each input.
Table 2-1
Input Guidance for LD50 ft-2 AnalysisApplication Type Formulation Input Guidance Broadcast Granular % incorporated 0 % Liquid Fl oz. Product/Acre This value is either reported on the product label or can be calculated from the application rate reported in other units (e.g., lbs a.i./Acre) on the label. Rows/Band/In-furrow Granular or Liquid Row Spacing Row spacing is the amount of space (inches) between crop rows and is obtained from the product label. If data are not on the product label then the assessor may contact the Biological and Economic Analysis Division (BEAD) for typical values for the crop being assessed. Band width Band width is the width of the applied pesticide row (inches) and is obtained from the product label. If data are not on the product label then the assessor may contact the Biological and Economic Analysis Division (BEAD) for typical values for the crop being assessed. % incorporated Value depends on the method of application: - T-Banded — covered with specified amount of soil: 99%
- In-furrow, drill, or shanked-in: 99%
- Side-dress, banded, mix, or lightly incorporate with soil: 85%
- Broadcast, mix, or lightly incorporated: 85%
- Side-dress, banded, unincorporated: 0%
- Broadcast, aerial broadcast, unincorporated: 0%
Liquid Fl oz. Product/Acre This value is either reported on the product label or can be calculated from the application rate reported in other units (e.g., lbs a.i./Acre) Results are described in Section 3 of this document.
-
2.4. Additional Inputs for Seed Treatment Analysis
Because of the differences in foliar application and seed treatment uses of pesticides, the seed treatment worksheet (Figure 2-4) can be used as a "stand-alone" tool for estimating avian and mammalian Risk Quotients (RQs) for the various crops listed. Efforts were made to make the crop list as complete as possible; however, additional crops may be added in the future as the need arises. If a crop is being assessed that is NOT included in T-REX, then the assessor should consult other sources to determine the proper seeding rate. Other sources may include extension agents, BEAD, registrants, and grower organizations. The source of the seeding rate value used in the assessment should be entered into T-REX and clearly documented in the assessment.
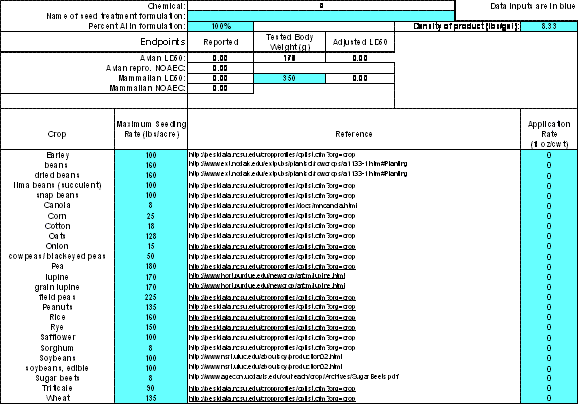 Figure 2-4
Figure 2-4
Example of seed treatment worksheetThe chemical name, along with the reported and adjusted LD50 and NOAEC values for birds and mammals will automatically be carried over from the Inputs spreadsheet. Additional data input cells (in blue) that need to be entered are described below:
Additional Input Cells Requiring Data Entry Name of Cell Entry Description Name of seed treatment formulation Enter the labels for seed treatment products. Seed treatment formulations differ from foliar applied formulations. Percent a.i. in formulation Enter the % Active Ingredient as a whole number (e.g., 24% = 24) Density of product (lbs./gal) Enter from chemical label. If unavailable, then the EFED default value is 8.33)
If a mammal species other than rat is used (e.g., dog, rabbit.), then enter the time-weighted average body weight of the animals that were tested in the experiment or reference weights obtained from a reliable source.Maximum Seeding Rate (lbs/acre) Maximum seeding rates are from the listed reference in T-REX (Cells C14 to C38) in the "seed treatments" worksheet. If values other than those included in T-REX are used, then the data source should be clearly entered and included in the assessment. If a commodity is being assessed that is NOT included in T-REX, then the assessor should consult other sources to determine the proper seeding rate. Other sources may include extension agents, BEAD, registrants, and grower organizations. The source of the seeding rate value used in the assessment should be entered into T-REX and clearly documented in the assessment. Application rate (fl oz./cwt) These data are obtained from the product label. Only data on commodities being assessed need to be entered. For example, if rye is not an intended use, then it should be set to zero, as this will have no impact on the RQ calculations for the other crops.
NOTE: If a liquid rate is not available for a chemical, enter the dry weight application rate (lbs a.i./cwt) in the adjoining cell. Once this is done, however, the underlying equation in that cell has been replaced. It is preferable that users input the fl oz/cwt value.
-
-
T-REX Calculations and Results
This section of the User’s Guide illustrates the T-REX output information that the user may incorporate into the assessment. The following figures are provided as an example only and the actual output will be dependent on the input parameters chosen from a specific usage pattern. The print area has been pre-set for each worksheet; therefore, "clicking" on the print button in the Excel tool bar will print user-friendly outputs. All EECs and RQ values are presented in yellow cells in T-REX. Estimated Environmental Concentrations (EECs), adjusted toxicity values, and risk quotients (RQs) are calculated based on data entered in the input worksheet. These values, as applied to risk estimation from foliar sprays (dietary residue analysis), seed treatment applications, and LD50 ft-2 analysis, are presented below.
-
3.1. Risk Estimation Based on Dietary Residue Concentrations (Foliar Spray)
The methods used by T-REX to estimate risk from consumption of selected contaminated food items are described below. For this analysis, T-REX calculates EECs and risk quotients based on both the upper bound and mean residue concentrations as presented by Hoerger and Kenaga (1972) and modified by Fletcher et al. (1994). These concentrations are determined using nomograms that relate application rate of a pesticide to residues remaining on dietary items of terrestrial organisms. The results of the upper bound and mean residue levels are presented in separate tabs ("upper bound Kenaga" and "mean Kenaga"); however, the methods used to calculate EECs and risk quotients are equivalent. Only RQs from the upper bound Kenaga worksheet should be used for comparison to levels of concern in the assessment. The mean residues, and the RQs generated from them, presented in the mean Kenaga worksheet are to be used only for risk description. Replacing the upper bound residues with the mean residues is not a valid mitigation approach when upper bound residues result in LOC exceedances. Based on the estimated dietary residue concentrations from the upper bound and mean Kenaga values, T-REX calculates the associated doses for various size classes of birds and mammals (Section 3.1.2). Both the dietary concentration (mg/kg-dietary item) and the resulting estimated doses (mg/kg-bw) may be used for risk estimation. The resulting dietary-based and concentration-based risk quotients are discussed in Section 3.1.4 of this User’s Guide.
This section describes how T-REX estimates the following:
- residue concentrations on selected food items (mg/kg-dietary item)
- dose-based EECs (mg/kg-bw) from dietary concentrations on selected food items
- adjusted toxicity values
- risk quotients
-
3.1.1. Calculation of Dietary Concentrations on Selected Food Items
The spreadsheet calculates the pesticide residue concentrations on each selected food item on a daily interval for one year. When multiple applications are modeled, residue concentrations resulting from the final application and remaining residue from previous applications are summed. The maximum concentration calculated (out of the 365 days) is returned as the EEC used to estimate potential risk to birds and mammals as described below. Dissipation of a chemical applied to foliar surfaces for single or multiple applications is calculated assuming a first-order decay rate from the following first-order rate equation:
Ct = C0e-kt
or in log form:
ln(Ct) = ln(C0)(-kT)
Where:
Ct = concentration, parts per million (ppm), at time T.
C0 = concentration (ppm), present initially (on day zero) on the surface of selected food items. C0 is calculated by multiplying the application rate, in pounds active ingredient per acre, by 240 for short grass, 110 for tall grass, and 135 for broad-leafed plants/small insects and 15 for fruits/pods/large insects for upper bound residue levels. Mean residue levels are derived by multiplying the application rate by 85 for short grass, 36 for tall grass, and 45 for broad- leafed plants/small insects and 7 for fruits/pods/seeds/large insects. Residue levels are based on work by Hoerger and Kenaga (1972) as modified by Fletcher et al. (1994). Additional applications are converted from pounds active ingredient per acre to ppm on the plant surface and the additional mass added to the mass of the chemical still present on the surfaces on the day of application.
k = Exponential rate constant = ln 2 ÷ foliar dissipation half-life. This value is in cell Q16 of the upper bound and mean Kenaga worksheets of T-REX. If the foliar dissipation data submitted to EFED are found scientifically valid and statistically robust for a specific pesticide, the 90% upper confidence limit of the mean half-lives should be used. When scientifically valid and statistically robust data are not available, EFED recommends using a default foliar dissipation half-life value of 35 days. The use of the 35- day half-life is based on the highest reported value (36.9 days), as reported by Willis and McDowell (1987).
t = time, in days, since the start of the simulation. The initial application is on day 0. The simulation is designed to run for 365 days.
The dietary concentrations estimated using the above methodology may be used directly to calculate risk quotients, but may also be used to calculate dose-based EECs (mg/kg-bw) for various size classes of mammals and birds as described in Section 3.1.2 below.
-
3.1.2. Calculating EEC Equivalent Doses Based on Estimated Dietary Concentrations on Selected Bird and Mammal Food Items
EECs (mg/kg-bw) for various size classes of mammals and birds may be calculated based on the dietary residue concentrations that are derived using the equations presented above. To allow for this type of analysis, the EECs and toxicity values are adjusted based on food intake and body weight differences so that they are comparable for a given weight class of animal. The size classes assessed are small (20-gram), medium (100-gram), and large (1000-gram) birds, and small (15-gram), medium (35-gram), and large (1000- gram) mammals. Equations used to calculate food intake (grams/day) and to adjust toxicity values for dose-based risk quotients are presented below.
Calculating Food Intake for Different Size Classes of Birds and Mammals
Daily food intake (g/day) is assumed to correlate with body weight using the following empirically derived equation (USEPA, 1993):
Avian consumption
F = (0.648 * BW0.651) / (1-W)
where:
F = food intake in grams of fresh weight per day (g/day)
BW = body mass of animal (g)
W = mass fraction of water in the food (EFED value = 0.8 for herbivores and insectivores, 0.1 for granivores)
Based on this equation, a 20-gram bird would consume 22.8 grams of food daily (114% of its body weight), a 100-gram bird would consume 65 grams of food daily (65% of its body weight daily), and a 1000-gram bird would consume 290 grams of food daily (29% of its body weight). These data, together with the residue concentrations (mg/kg-food item) on selected food items calculated from the Kenaga nomogram, are used to estimate the dose (mg/kg-bw) of residue consumed by the three size classes of birds as discussed below. Using a small (20-gram) bird as an example, a dietary concentration of 100 mg/kg-diet (ppm) x 1.14 kg diet/kg bw (114%) would result in an equivalent dose-based EEC of 114 mg/kg-bw. T-REX calculates food intake based on dry weight and wet weight of food items. The dose-based assessment uses the wet weight food consumption values by assuming that dietary items are 80% water by weight. However, if dietary items of a species that is being assessed are known, then a refined dose-based EEC can be calculated using appropriate water fractions of the food items.
A similar relationship between body weight and food intake has been derived for mammals (USEPA 1993):
Mammalian food consumption (g/day)
F = (0.621 * BW0.564) / 1 - W)
where:
F = food intake in grams of fresh weight per day (g/day)
BW = body mass of animal (g)
W = mass fraction of water in the food (EFED value = 0.8 for herbivores and insectivores, 0.1 for granivores)
The scaling factors, which result in a percent body weight consumed, are presented in the following table for each weight class of mammal. These values are used in the same manner as birds in calculating dose-based EECs (mg/kg-bw). Note the difference in food intake of granivores compared with herbivores and insectivores. This is caused by the difference in the assumed mass fraction of water in their diets.
Difference in Food Intake of Granivores versus Herbivores and Insectivores Organism and body weight Food intake (g day-1)a Percent body weight consumed (day-1)a Herbivores / Insectivores Granivores Herbivores / Insectivores Granivores 15 g 14.3 3.2 95 21 35 g 23 5.1 66 15 1000 g 150 34 15 3 aThe first number in this column is specific to herbivores/insectivores. The second number is for granivores. These groups have markedly different consumption requirements.
T-REX calculates food intake based on dry weight and wet weight of food items (wet weight is used for RQ calculations). The dose-based assessment uses the wet weight food consumption values by assuming that dietary items are 80% water by weight (10% for granivores). However, if dietary items of a species being assessed are known, then a refined dose-based EEC can be calculated using appropriate water fractions of the food items.
-
3.1.3. Calculating Adjusted Toxicity Values
The dose-based EECs (mg/kg-bw) derived above are compared with LD50 or NOAEL (mg/kg-bw) values from acceptable or supplemental toxicity studies that are adjusted for the size of the tested animal compared with the size of the animal being assessed (e.g., 20-gram bird). These exposure values are presented as mass of pesticide consumed per kg body weight of the animal being assessed (mg/kg-bw). EECs and toxicity values are relative to the animal’s body weight (mg residue/kg bw) because consumption of the same mass of pesticide residue results in a higher body burden in smaller animals compared with larger animals. For birds, only acute values (LD50 s) are adjusted because dose-based risk quotients are not calculated for the chronic risk estimation. Adjusted mammalian LD50s and reproduction NOAELs (mg/kg-bw) are used to calculate dose-based acute and chronic risk quotients for 15-, 35-, and 1000-gram mammals. The following equations are used for the adjustment (USEPA 1993):
Adjusted avian LD50:
Adj. LD50 = LD50 (AW / TW)(x-1)
where:
Adj. LD50 = adjusted LD50 (mg/kg-bw) calculated by the equation
LD50 = endpoint reported from bird study (mg/kg-bw)
TW = body weight of tested animal (178g bobwhite; 1580g mallard; 350g rat)
AW = body weight of assessed animal (avian: 20g, 100g, and 1000g)
x = Mineau scaling factor for birds; EFED default 1.15
Adjusted mammalian NOAELs and LD50s (note that the same equation is used to adjust the NOAEL):
Adj. NOAEL or LD50 = NOAEL or LD50 (TW / AW)(0.25)
where:
Adj. NOAEL or LD50 = adjusted NOAEL or LD50 (mg/kg-bw)
NOAEL or LD50 = endpoint reported from bird study (mg/kg-bw)
TW = body weight of tested animal (350g rat)
AW = body weight of assessed animal (15g, 35g, 1000g)
-
3.1.4. Calculating Risk Quotients
Two types of risk quotients are calculated by T-REX based on the estimated dietary residue concentrations determined from the Kenaga nomogram: dietary based RQs and dose based RQs. These RQs are not equivalent. Dietary risk quotients are calculated by directly comparing the concentration of an administered pesticide (or estimated to be administered) to experimental animals in the diet in a toxicity study to the concentration estimated on selected food items. These risk quotients do not account for the fact that smaller sized animals need to consume more food relative to their body weight than larger animals or that differential amounts of food are consumed depending on the water content and nutritive value of the food. The dose- based risk quotients do account for these factors. The dose-based RQs incorporate the ingestion rate-adjusted exposure from the various food items for the different weight classes of birds and the weight class-scaled toxicity endpoints. Formulas presented in Table 3-1 are used to calculate dose-based and dietary-based risk quotients:
Table 3-1
Formulas used to calculate dose- and dietary-based risk quotientsDuration Dose or Dietary RQ Surrogate Organism Equation Acute Dose-based Birds and mammals Acute Daily Exposure (mg/kg-bw) / adjusted LD50 (mg/kg-bw) Dietary-based Birds Kenaga EEC (mg/kg-food item) / LC50 (mg/kg-diet) Chronic Dietary-based Birds and mammals EEC (mg/kg-food item) / NOAEC (mg/kg-diet) Dose-based Mammals only EEC (mg/kg-bw) / Adjusted NOAEL (mg/kg-bw) These risk quotients are compared to the Agency’s LOCs to determine if risk is greater than those levels of concern.
-
3.1.5. Graphs
The last section of the upper bound and mean Kenaga worksheets displays the terrestrial residues graph (Figure 3-1). The user can copy/paste this graph into a word processor document for use in the assessment. The accumulation and decline of the pesticide residues on each of the four food items are plotted over the first 100 days after the initial application. The "Graphs" worksheet provides similar graphs, but also includes mammalian and avian LOCs to allow the assessor to display the magnitude and duration of LOC exceedances in the assessment. These data are useful to include for risk discussion purposes.
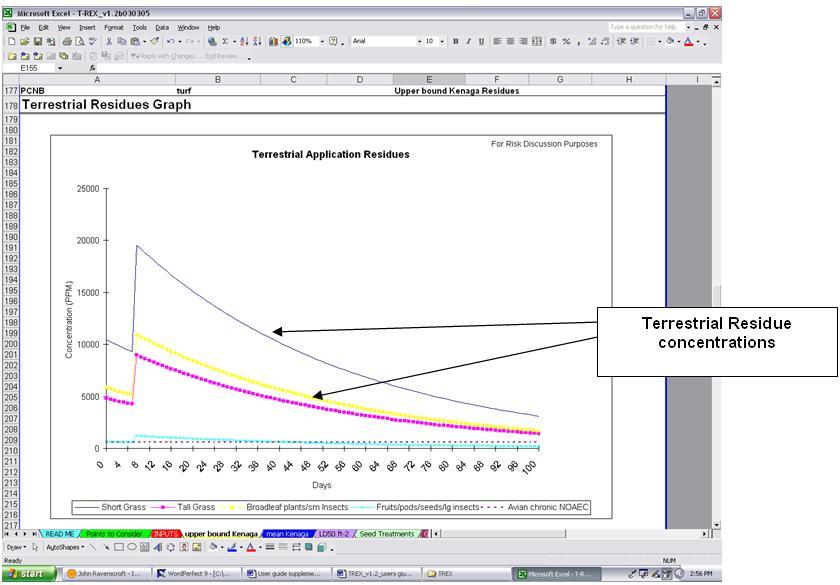 Figure 3-1
Figure 3-1
Kenaga residue worksheet (residue graph section)
-
3.2. Results of LD50 ft-2 Analysis
Results of the LD50 ft-2 analysis are presented in the "LD50 ft-2" tab. Simply click on the LD50 ft-2 tab to display this page (Figure 3-2). There are three main sections in this worksheet: the input summary, row/band/in- furrow application summary, and broadcast application summary. The input summary reiterates the parameters supplied by the user on the "input" worksheet. The other two sections calculate LD50 ft-2 values for row/band/in-furrow applications and broadcast applications, respectively. The calculations are based on a toxicity (adjusted LD50) and exposure (mg a.i./ft2) value. (Adjusted LD50s are discussed in Section 3.1.3). Equations used to calculate EECs are presented below for broadcast and banded granular and liquid applications. Percent incorporation in these equations for various application types entered by the assessor into T-REX is presented in Table 3-2.
Banded granular applications
mg a.i. ft-2 = (application rate x % a.i. x 453,590 mg/lb)/(no. of rows A-1 x row length x bandwidth)
exposed mg a.i. ft-2 = mg a.i. ft-2 x % unincorporation
Banded liquid applications
mg a.i. ft-2 = (mg a.i. 1000 ft-1 row)/(1000 ft x bandwidth)
exposed mg a.i. ft-2 = mg a.i. ft-2 x % unincorporation
Broadcast granular applications
mg a.i. ft-2 = (application rate x % a.i. x 453,590 mg/lb)/43,560 ft2 acre-1
Broadcast liquid applications
mg a.i. ft-2 = (fl oz product A-1 x 28349 mg/oz x % a.i.)/43,560 ft2 acre -1
Table 3-2
Percent unincorporated assumed for several types of applications.% incorporated - T-Banded – covered with specified amount of soil: 1%
- in-furrow, drill, or shanked-in: 1%
- Side-dress, banded, mix, or lightly incorporate with soil: 15%
- Broadcast, mix, or lightly incorporated: 15%
- Side-dress, banded, unincorporated: 100%
- Broadcast, aerial broadcast, unincorporated: 100%
Based on these EECs, the LD50 ft-2 is calculated using the following equation:
LD50 ft-2 = EEC (mg a.i./ft-2 ) / (Adj LD50 ÷ bw (kg) of assessed animal)
The LD50/ft2 is then compared with the Agency's LOCs in an analogous way in which risk quotients are compared.
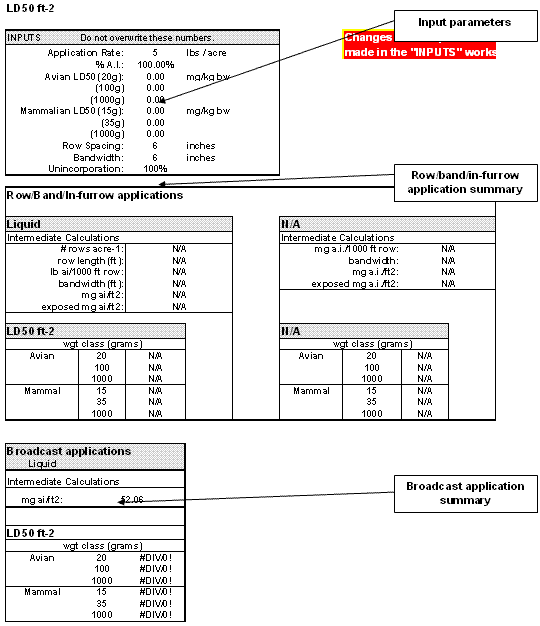 Figure 3-2
Figure 3-2
LD50 per square foot worksheetThe worksheet "Granular Characterization Calcs" calculates the number of granules that need to be consumed by a bird to achieve a dose that would exceed the adjusted LD50 and trigger the endangered species LOC of 0.1 and the acute LOC of 0.5 (Figure 3-3). There are two inputs (blue cells): weight of an assessed bird and weight of a single granule. Weight of an assessed bird is typically 20 grams (0.02 kg), but any weight may be entered. In addition, the minimum foraging area with sufficient number of granules to achieve a dose that exceeds the adjusted LD50 or 1/10th the adjusted LD50 is estimated by assuming that a bird consumes 100%, 50%, and 10% of the available granules.
Figure 3-3
Granular Characterization Calcs WorksheetEstimation of the number of granules needed to achieve toxicity thresholds
Step 1
Estimate mass of a.i. consumed for the assessed species weight to achieve the desired toxicity thresholdParameter Value Comment Bird Mammal Weight of assessed bird (kg) 0.10 0.015 INPUT (0.02 kg and 0.015 kg are typically used for general assessments for birds and mammals, respectively). Adjusted LD50, mg/kg-bw 0.00 0.00 Calculated from the body weight entered in B13 and C13 and LD 50 entered in the "Inputs" sheet. Mg a.i. needed to achieve the adjusted LD50 for bird of assessed weight 0.00 0.00 mg a.i./kg-bw * kg-bw = mg a.i Mg a.i. needed to achieve acute LOC exceedance (1/2 adjusted LD 50) for bird of assessed weight 0.00 0.00 Mg a.i. needed to achieve endangered species LOC exceedance (1/10th adjusted LD50) for bird of assessed weight 0.00 0.00 Step 2
Determine the mass of a.i. per granuleParameter Value Comment Bird Mammal Percent of a.i. in formulated product Must enter percent a.i. specified on the label Weight of 1 granule (mg, obtained from registrant) INPUT (Note:Be sure to enter units in mg!) mg a.i./granule 0.0000 0.0000 weight of granule (mg) x fraction of a.i. = mg a.i./granule Step 3
Calculate number of granules with mass of a.i. equivalent to adjusted LD50 for bird of assessed weightParameter Value Comment Bird Mammal No. of granules needed to achieve adjusted LD50 #DIV/0! #DIV/0! mg a.i. needed to achieve adjusted LD50 (B/C14) / mg a.i. per granule (B/C20) No. of granules needed to achieve acute LOC exceedance (1/2 adjusted LD50 #DIV/0! #DIV/0! mg a.i. needed to achieve 1/2 adjusted LD50 (B/C15) / mg a.i. per granule (B/C20) No. of granules needed to achieve endangered species LOC exceedance (1/10 adjusted LD50) #DIV/0! #DIV/0! mg a.i. needed to achieve 1/10 adjusted LD50 (B/C16) / mg a.i. per granule (B/C20) Minimum Foraging Area Needed to Allow for Ingestion of Sufficient Mass of a.i. to Achieve LOC Exceedance Parameter Value Comment Bird Mammal EEC (mg a.i./square foot) 52.06 52.06 From LD50 ft-2 page Foraging area (square feet) needed to achieve endangered species LOC exceedance assuming 100% feeding efficiency 0.00 0.00 mg a.i. needed to achieve LD50 in 20-gram bird (B/C16) / mg a.i. per sq. ft (B/C27) Foraging area (square feet) needed to achieve endangered species LOC exceedance assuming 50% feeding efficiency 0.00 0.00 Foraging area needed to achieve LOC Exceedance assuming 100% feeding efficiency (B/C28) * 2 (i.e., twice the foraging area is needed for 50% feeding efficiency) Foraging area (square feet) needed to achieve endangered species LOC exceedance assuming 10% feeding efficiency 0.00 0.00 Foraging area needed to achieve LOC Exceedance assuming 100% feeding efficiency (B/C28) * 10 (i.e., 10 times the foraging area is needed for 10% feeding efficiency) -
3.3. Results of Seed Treatment Applications
The seed treatment worksheet is organized into three main areas: data input, intermediate calculations, and risk quotients. For each of the commodities for which application rates are entered, values of exposure and dose are displayed in the intermediate calculations section (Figures 3-4 and 3-5). The application rates of the active ingredient (a.i.) and of seed are presented in the first two columns, while the doses to birds and mammals and the exposed a.i. are displayed in the last three columns. The scaling factors used to adjust the reported LD50 to a 20 g bird and a 15 g mammal are also displayed for the risk assessor. The specific equations used for this adjustment were previously discussed (Section 3.1.3.).
The seed treatment worksheet calculates the avian and mammalian dose (mg a.i. bw -1 day-1), available pesticide (mg a.i. ft-2). The acute and chronic RQs for birds and mammals as described below.
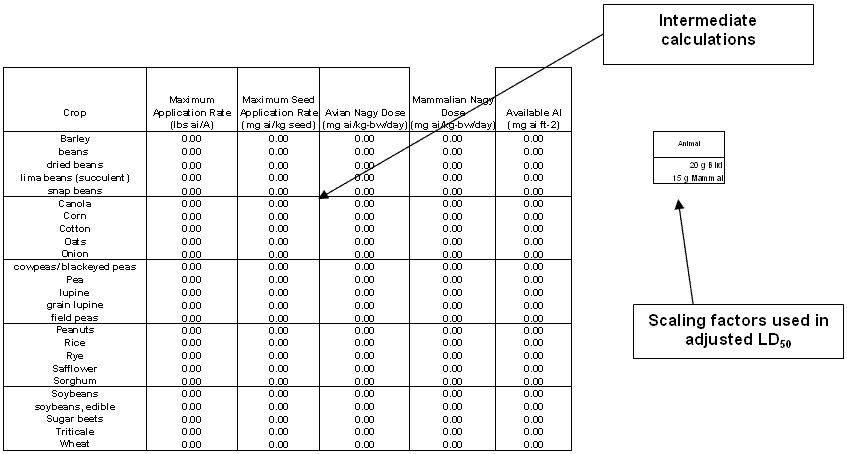 Figure 3-4
Figure 3-4
Seed treatment worksheet (intermediate calculations section) Figure 3-5
Figure 3-5
Seed treatment worksheet (avian and mammalian RQs)-
3.3.1. Calculation of Avian and Mammalian Doses (EECs)
As previously discussed, doses from seed treatment applications are calculated using the scaling factor approach for a 20-gram bird and a 15-gram mammal using the following general equation:
Dose (mg/kg a.i. day-1) = (maximum seed application rate (mg a.i. kg seed-1) x scaling factor) / body weight of assessed animal.
Where:
Maximum Seed Application Rate (mg a.i./kg seed) = (Application rate x 10,000)
- Application Rate (lbs a.i./cwt) = ( (Application rate (fl oz/cwt) x fraction of a.i. in formulation) / 128 fl oz/gallon) x density of product (lbs/gallon)
- Maximum Application Rate (lbs a.i./A) = (Maximum seeding rate x application rate (lbs a.i./cwt)) / 100 lbs/cwt
Scaling factor:
Equations presented in USEPA (1993) are used to adjust food intake and toxicity values to account for the differences in the size of the assessed animal compared with the size of the animal used in the toxicity tests. The doses used as the basis for risk quotient calculations are determined using the following equations and are referred to as Avian and Mammalian Nagy Doses in the seed treatment tab of T-REX:
Avian and Mammalian Nagy Dose (mg a.i./kg-bw) = (daily food intake g/day x 0.001 kg/g x maximum seed application rate (mg/kg-seed) / body weight of animal (kg)
Available a.i. (mg a.i./ft2) = The amount of available pesticide is calculated by converting the maximum application rate (lbs acre-1) to mg a.i./ft2) using the following equation: (Maximum application rate (lbs/Acre) x 106 mg/kg) / (43,560 square feet/acre x 2.2 lb/kg)
-
3.3.2. Calculation of Risk Quotients for Seed Treatment Applications
RQs are calculated using the adjusted LD50 for the smallest weight class of animal (20 g for birds and 15 g for mammals), using methodology previously discussed and assuming a tested body weight of 178 g for quail, 1580 g for duck, and 350 g for the lab rat. Acute RQs are calculated using two methods:
Method 1 (EEC/LD50 method)
Acute RQ = mg a.i./kg-1 day-1/adjusted LD50
Method 2 (analogous to the LD50 ft-2 method)
Acute RQ = mg/kg-1 a.i. ft-2/ (adjusted LD50 x body weight (kg))
These risk quotients are compared to the Agency's levels of concern for risk estimation. The appropriate risk quotient will be chemical specific although risk quotients derived from each method may be used to characterize risk.
Chronic RQs are calculated using the following equation:
Chronic RQ = mg a.i. kg-1 seed / NOAEC (mg/kg-diet).
Chronic RQs are not adjusted to allow for an assessment of risk to different weight classes of birds or mammals.
-
-
3.4. Printing Results
The results from the upper bound Kenaga, mean Kenaga, and LD50 ft-2 sheets are summarized in a separate worksheet "Print Results." This worksheet is designed so that the individual tables may be easily cut and pasted directly into Microsoft Word documents with minimal formatting changes. This sheet is password protected; however, formatting changes (e.g., changing significant digits) are allowed. This worksheet includes all data that is included in the risk quotient calculations (EEC, Toxicity Value, and Risk Quotient).
-
-
Risk Description Points to Consider
-
4.1. Acute and Reproduction Dietary Discussions
The risk assessment includes numerous calculations of dietary exposure for multiple weight classes of animals. However, there are energetic considerations that suggest that some weight class/food item combinations are not likely to naturally occur. For example, there are not likely to be many 15 g mammals or 20 g birds that exclusively feed on vegetation. The risk assessor is urged to consult such texts as the Wildlife Exposure Factors Handbook (USEPA 1993), which will provide more comprehensive approaches for considering energy requirements and energy availability to estimate dietary exposure. In addition, the age of individuals may also play an important role in the types and relative amounts of food items selected. This factor should also be taken into account when describing dietary risks.
-
4.2. Acute Toxicity RQ Approaches
Dose-based and dietary-based acute RQs should be provided to risk managers whenever effects data allow. The dose-based approach assumes that the uptake and absorption kinetics of a gavage toxicity study approximate the absorption associated with uptake from a dietary matrix. Toxic response is a function of duration and intensity of exposure. Absorption kinetics across the gut and enzymatic activation/deactivation of a toxicant may be important and are likely variable across chemicals and species. For many compounds, a gavage dose represents a very short-term high intensity exposure, whereas dietary exposure may be of a more prolonged nature. The dietary-based approach assumes that animals in the field are consuming food at a rate similar to that of confined laboratory animals. Energy content in food items differs between the field and the laboratory and so do the energy requirements of wild and captive animals. The Wildlife Exposure Factors Handbook can provide insights into energy requirements of animals in the wild as well as energy content of their diets (USEPA, 1993).
-
4.3. Reproduction RQ Approach
The typical 21-week avian reproduction study does not define the true exposure duration needed to elicit the observed responses. The study protocol was designed to establish a steady-state tissue concentration for bioaccumulative compounds. For other pesticides, it is entirely possible that steady-state tissue concentrations are achieved earlier than the 21-week exposure period. Moreover, pesticides may exert effects at critical periods of the reproduction cycle; therefore, long-term exposure may not be necessary to elicit the effect observed in the 21-week protocol. The EFED screening-level risk assessment uses the single-day maximum estimated EEC as a conservative approach. The degree to which this exposure is conservative cannot be determined by the existing reproduction study. However, risk assessment discussions should be accompanied by the graphics from the T-REX model regarding the number of days dietary exposure is above the NOAEC. The greater number of days EECs exceed the NOAEC, the greater the confidence in predictions of reproductive risk concerns.
-
References
Dunning, J.B. 1984. Body weights of 686 species of North American birds. Western Bird Banding Assoc. Monograph No. 1.
Fletcher, J.S., J.E. Nellesson and T. G. Pfleeger. 1994. Literature review and evaluation of the EPA food-chain (Kenaga) nomogram, an instrument for estimating pesticide residues on plants. Environ. Tox. And Chem. 13(9):1383-1391.
Hoerger, F. and E.E. Kenaga. 1972. Pesticide residues on plants: correlation of representative data as a basis for estimation of their magnitude in the environment. IN: F. Coulston and F. Corte, eds., Environmental Quality and Safety: Chemistry, Toxicology and Technology. Vol 1. George Theime Publishers, Stuttgart, Germany. pp. 9-28.
Mineau, P., B.T. Collins, A. Baril. 1996. On the use of scaling factors to improve interspecies extrapolation to acute toxicity in birds. Reg. Toxicol. Pharmacol. 24:24-29.
Urban, D. J. 2000. Guidance for conducting screening-level avian risk assessments for spray applications of pesticides. OPP/EFED, USEPA. July 7, 2000.
USEPA. 1992. Comparative analysis of acute avian risk from granular pesticides. Office of Pesticide Programs. USEPA. March 1992.
USEPA. 1993. Wildlife exposure factors handbook. Volume I of II. EPA/600/R-93/187a. Office of Research and Development, Washington, D. C. 20460.
USEPA. 1995. Great Lakes water quality technical support document for wildlife criteria. Office of Water, Washington D.C. Document Number EPA-820-B095-009.
Willis and McDowell. 1987. Pesticide persistence on foliage. Environ. Contam. Toxicol. 100:23-73.
T-REX FORMULAS (Version 1.4.1)
Converting NOAEC to/from NOAEL
If reported chronic endpoint is mg/kg-diet (NOAEC) in a laboratory rat, then divide by 20 to get mg/kg-bw.
If the reported chronic endpoint is mg/kg-bw (NOAEL) in a laboratory rat, then multiply by 20 to get mg/kg-diet.
Adjusted LD50 (and also adjusted NOAEL for mammals)
The LD50 values entered on the input form are adjusted for animal class (20, 100 and 1000 g birds and 15, 35, and 1000 g mammals), using the following equations: (The adjusted mammalian NOAEL uses the same equation as the adjusted mammal LD50).
- Avian LD50
Adj. LD50 = LD50 (AW / TW)(x-1)
- Mammal LD50
Adj. LD50 = LD50 (TW / AW)(0.25)
- Mammal NOAEL
Adj. NOAEL = NOAEL (TW / AW)(0.25)
where:
Adj. LD50 = adjusted LD50
LD50 = acute endpoint reported from bird or mammal study
TW = body weight of tested animal (178 g bobwhite; 1580 g mallard; 350 g rat)
AW = body weight of assessed animal (avian: 20 g, 100 g, 1000 g; mammals: 15 g, 35 g, 1000 g)
x = Mineau scaling factor for birds; EFED default 1.15
Scaling Factors
The scaling factors (USEPA, 1993) used in the consumption-weighted EECs are:
- Avian consumption
F = (0.648 * BW0.651) / (1 - W)
- Mammal consumption
F = (0.621 * BW0.564) / (1 - W)
where:
F = food intake in grams of fresh weight per day
BW = body mass of animal (avian: 20 g, 100 g, 1000 g; mammal: 15 g, 35 g, 1000 g)
W = mass fraction of water in the food (0.8 for herbivores and insectivores, 0.1 for granivores)
Once the daily food intake values have been calculated using the scaling factors, the next step is to calculate what percent of the overall body weight this consumed food intake represents for each weight class.
RQ formulas Using Upper Bound Kenaga (EEC) Residues or Mean Kenaga Residues
EEC equivalent dose (mg/kg-bw) = upper bound EEC * (% body weight consumed/100)
Avian
Dose-based RQs = EEC equivalent dose / adjusted LD50
Dietary-based RQs
- Acute: EEC / LC50
- Chronic: EEC / NOEAC
Mammal
Dose-based RQs = EEC equivalent dose / adjusted N0AEL
Dietary-based RQs
- Acute: EEC / LC50
- Chronic: EEC / NOEAC
LD50 ft-2
Exposure Values
- Banded granular applications
mg a.i. ft-2 = (application rate x % a.i. x 453,590 mg/lb)/(no. of rows A-1 x row length x bandwidth)
exposed mg a.i. ft-2 = mg a.i. ft-2 x % unincorporation
- Banded liquid applications
mg a.i. ft-2 = (mg a.i. 1000 ft-1 row)/(1000 ft x bandwidth)
exposed mg a.i. ft-2 = mg a.i. ft-2 x % unincorporation
- Broadcast granular applications
mg a.i. ft-2 = (application rate x % a.i. x 453,590 mg/lb)/43,560 ft2 acre-1
- Broadcast liquid applications
mg a.i. ft-2 = (fl oz product A-1 x 28349 mg/oz x % a.i.)/43,560 ft2 acre-1
LD50 ft-2 Calculations
LD50 ft-2 (Avian) = (Exposed mg a.i. ft-2) / (Adjusted LD50 * .02)
LD50 ft-2 (Mammal)= (Exposed mg a.i. ft-2) / (Adjusted LD50 * .015)
Seed Treatments
The seed treatment worksheet calculates the avian and mammalian dose (mg a.i./kg-bw day-1), available pesticide (mg a.i. ft-2), and acute and chronic RQs for birds and mammals.
Adjusted LD50s are calculated as indicated previously. Only a 20 g bird and a 15 g mammal are assessed. The tested body weights are as follows: 178 g for quail, 1580 g for duck, and 350 g for the lab rat.
As discussed previously, doses are calculated using the scaling factor approach.
Maximum Seed Application Rate (mg a.i./kg seed) = (Application rate x 2.2 x 106) / (100 x 2.2) = (Application rate x 10,000)
- Application Rate (lbs a.i./cwt) = (Application rate (fl oz/cwt) x decimal % of a.i. in formulation) / 128 fl oz/gallon) x density of product (lbs/gallon)
- Maximum Application Rate (lbs a.i./A) = (Maximum seeding rate x application rate (lbs a.i./cwt)) / 100 lbs/cwt
Avian and Mammalian Nagy Dose (mg a.i./kg-bw) = (daily food intake g/day x 0.001 kg/g x maximum seed application rate (mg/kg-seed) / body weight of animal (kg)
Available a.i. (mg a.i./ft2) = The amount of available pesticide is calculated by converting the maximum application rate (lbs acre-1) to mg a.i./ft2), using the following equation: (Maximum application rate (lbs/Acre) x 106 mg/kg) / (43,560 square feet/acre x 2.2 lb/kg)
Acute and Chronic RQs
- Acute RQ #1 = (Avian or Mammal) Nagy Dose / (adjusted LD50)
- Acute RQ #2 = Available a.i. / (Adjusted LD50* kg body weight)
- Chronic RQ = Maximum Seed Application Rate / NOAEC
Granular Characterization Calculations
Calculations are presented in Column D of the "Granular Characterization Calcs" worksheet of T-REX 1.4.1.
