
| Great Lakes Ecosystems | ||

|
|
|||||||||
|
1994
Proceedings
|
|
EVOLUTIONARY MODELS OF PLANT POPULATION/COMMUNITY
DYNAMICS AND CONSERVATION OF SOUTHEASTERN PINE SAVANNAS William J. Platt |
 |
Models of change over ecological periods of time as a result of natural processes traditionally have been based on concepts of succession (Fig. 1). Large-scale disturbances are hypothesized to disrupt existing species associations, reinitiating autogenic processes (Tansley 1935). Over time, natural systems are postulated to approach an equilibrium (or at least the scale of change diminishes) until large-scale disturbances reinitiate succession (see review of Connell and Slatyer 1977). Non-catastrophic disturbances that recur within the lifespans of species in a given seral stage have been hypothesized to select for traits that enhance survival (Whittaker 1953). Such species are postulated not to be replaced in such habitats as long as the non-catastrophic disturbances are chronic, producing a subclimax state (Fig. 1).
This classical model of succession, developed primarily in midwestern and northeastern habitats, has been applied to southeastern habitats in which fire is recognized as a major chronic disturbance (Fig. 2). For example, during the past half-century, frequent fires have been hypothesized to maintain pine savanna/scrub subclimaxes in sandhills (Wells 1928; Chapman 1932a,b; Laessle 1958; Monk 1968). Differences in characteristics of fire regimes have been ignored; lightning and/or native Americans have been suggested as the agents (e.g. Quarterman and Keever 1962). Characteristics of vegetation to withstand (e.g., longleaf pine) or to reproduce (e.g., sand pine) in the post-fire environment have been postulated as adaptations resulting from different fire frequencies. Models have been developed that predict that fire frequency determines the occurrence of different types of subclimax communities (longleaf pine savannas or sand pine scrubs), as well as climax (hardwood forest) communities (Myers 1985; Myers and White 1987; Stout and Marion 1993).
These models have not successfully predicted long-term patterns of change in vegetation when fire is excluded from southeastern pine savannas and scrub (Marks and Harcombe 1981; Abrahamson 1984; Peroni and Abrahamson 1986; Menges et al. 1993). Internal changes occur with fire suppression, as for example in sandhills, where prominence of oaks increases relative to pines (Veno 1976; Marks and Harcombe 1981; Gilliam et al. 1993), but these internal changes are reversible once "natural" fire regimes are reintroduced (Streng et al. 1994; Glitzenstein and Platt 1994). Shifts towards putative climax communities also have not been documented even when periods of fire exclusion approach, in some cases of scrub in the Florida panhandle, two centuries (Platt and Doyle 1994). These studies are bolstered by the apparent absence of intermediate seral stages or upland climax communities in presettlement vegetation (Schwartz 1994), although there are suggestions of variation in the pine and oak dominated communities in the presettlement vegetation (Platt and Schwartz 1990; Harcombe et al. 1994).
I propose a three stage evolutionary model in which characteristics of both plant species and disturbances change non-independently over time (Fig. 2). In environments in which disturbances are chronic and the post-disturbance environment is not fatal, traits that confer resistance (survival of some life cycle stage) will be favored. Once resistance occurs, traits conferring increased fitness in post-disturbance environments will be favored, producing adaptation to and even dependence on the disturbance. Third, traits that modify the disturbance regime in ways that result in increased fitness would be favored. This pattern can be repeated in a manner similar to plant-herbivore co-evolution (see Howe and Westley 1988) as long as the chronic nature of the disturbance is not interrupted.
Proposed patterns to community evolution by resistance-adaptation-modification can be explored using longleaf pine savannas and lightning-season fires as an exemplary system. Longleaf pine itself is well known as the most fire resistant tree in the southeast (Chapman 1932a,b; Wahlenberg 1946). The thick, layered bark results in large trees surviving fires (Chapman 1932a). Needles of juvenile grass-stage pines burn, but the buds are resistant to fire (Chapman 1932a,b; Boyer 1974; Croker and Boyer 1975). Indeterminate flushing of needles from these buds during the growing season enables burned juveniles to survive fires (Wahlenberg 1946). Moreover, this fire resistance of juveniles appears as early as the second year after germination (Grace and Platt 1994). Such fire-resistant traits result in highly uneven ages of trees in old-growth longleaf pine stands (Platt et al. 1988b; Platt and Rathbun 1994).
Ground cover plants in longleaf pine savannas burn, but the growth forms greatly reduce the chances that perennating buds will be harmed. Most species are adapted for post-fire environments (e.g., Wells and Shunk 1931). Flowering often occurs profusely following growing season fires, and many herbaceous species have become dependent on these fires (Lemon 1949; Platt et al. 1988a; 1991; Streng et al. 1994). Some of these species have become even more narrowly adapted, flowering and/or spreading clonally primarily following growing season fires (e.g. Aristida beyrichiana, Streng et al. 1994; Pityopsis graminifolia, Brewer and Platt 1994a,b).
Species in longleaf pine savannas also modify the frequency, intensity, and timing of fire regimes. Mutch (1970) first hypothesized that flammable traits increase the likelihood of fire. Longleaf pine needles are flame retardant when green, but retention times for needles on trees are short (2 years), and needles are shed annually (Landers 1991). Once shed, pine needles are incendiary; maximum fire temperatures increase as the density of pines or pine needles increases (Platt et al. 1991; Grace and Platt 1994). In addition, longleaf pine also appears to enhance the initiation of fires via translation of lightning strikes into ground fires, especially where the ground cover consists of flammable herbs (Platt et al. 1988b; Platt et al. 1991; Landers 1991).
Pyrogenicity could shape the landscape and ultimately influence evolution of indigenous species. For example, shedding of needles prior to the spring would enhance occurrence of fires at times of maximum impact on hardwoods. Topkill (and complete kill) of oaks is much greater in the spring than at other times of the year (Glitzenstein and Platt 1994). Oaks (e.g., turkey oak, Quercus laevis; bluejack oak, Quercus incana) indigenous to these habitats remain in the ground cover until there are no fires for a period of time and/or until pines are not present above them in the overstory (Rebertus et al. 1989, 1994). Some hardwoods (e.g., runner oaks: Quercus minima, Q. pumila) have abandoned tree habits entirely, suggesting adaptation to modifications of fire regimes produced by pines and other ground cover vegetation.
No equilibrium plant communities are predicted by this evolutionary model. Instead, disturbance regimes and species change continually over time, with limits to such "co-evolution" likely to depend on the variability inherent in the disturbance and the indigenous plant populations (and ultimately, by large-scale environmental changes). Nonetheless, evolutionary histories become unique pathways dependent on the species and their interactions with the disturbance, with the disturbance regime becoming integral to the environment. Community and landscape characteristics such as an open savanna dominated by longleaf pines and herbaceous species that produce a highly pyrogenic fine fuel thus can best be considered a product of community evolution that has occurred over some extended period of time.
Major novel disruptions could easily destroy unique environments created by "co-evolution" of plants and disturbances. For example, southeastern pine savanna environments have been greatly altered by anthropogenic disturbances, including open-range grazing, clear-cutting of pines, and most recently, extensive fire suppression (Ware et al. 1993). The hypothesized self-perpetuating nature of longleaf pine savannas would not have persisted following such impacts (although changes occurred slowly where anthropogenic fire was maintained). Those changes that followed extensive anthropogenic alterations (i.e., replacement of pine savannas by hardwood stands; see Heyward 1939) apparently misled biologists into thinking that such changes were natural successional processes, although what was perceived as succession actually was not integral to the system prior to human intervention.
Models for invasion by exotics may be more appropriate than successional models for describing changes in "coevolved" systems (such as southeastern pine savannas) following anthropogenic disruptions. Such systems may be particularly susceptible to invasions by "exotics" once environmental conditions characterizing the original habitat no longer are present. Conservation efforts will benefit from models in which traits that facilitate invasion of perturbed systems by "exotics" are related to the dynamics of invasion by such "exotics" (non fire-adapted species) and the resulting alterations of habitats produced by these species (see Hengeveld 1994).
Abrahamson, W. G. 1984. Post-fire recovery of Florida Lake Wales Ridge vegetation. American Journal of Botany 71:9-21.
Boyer, W. D. 1974. Impact of prescribed fires on mortality of released and unreleased longleaf pine seedlings. U.S. Forest Service Research Note SO-128. Southern Forest Experiment Station, New Orleans, Louisiana.
Brewer, J. S. and W. J. Platt. 1994. Effects of fire season and herbivory on reproductive success in a clonal forb, Pityopsis graminifolia (Michx.) Nutt. Journal of Ecology (in press).
Chapman, H. H. 1932a. Is the longleaf type a climax? Ecology 13:328-334.
Connell, J. H. and R. O. Slatyer. 1977. Mechanisms of succession in natural communities and their role in community stability and organization. The American Naturalist 111:1119-1144.
Croker, T. C., Jr. and W. D. Boyer. 1975. Regenerating longleaf pine naturally. U.S. Forest Service Research Paper SO-105. Southern Forest Experiment Station, New Orleans, Louisiana.
Gilliam, F. S., B. M. Yurish, and L. M. Goodwin. 1993. Community composition of an old growth longleaf pine forest: relationship to soil texture. Bulletin of the Torrey Botanical Club 120:287-294.
Glitzenstein, J. S. and W. J. Platt. 1994. Effects of fire regime and habitat on tree dynamics in north Florida longleaf pine savannas. Ecological Monographs (in press).
Grace, S. L. and W. J. Platt. 1994. Effects of adult tree density and fire on the demography of pre-grass stage juvenile longleaf pine (Pinus palustris Mill.). Journal of Ecology (in press).
Harcombe, P. A., J. S. Glitzenstein, R. G. Knox, S. L. Orzell, and E. L. Bridges. 1994. Vegetation of the longleaf pine region of the west Gulf coastal plain. Proceeding Tall Timbers Fire Ecology Conference (in press).
Hengeveld, R. 1994. Small-step invasion research. Trends in Ecology and Evolution 9:339-342.
Heyward, F. 1939. The relation of fire to stand composition of longleaf pine forests. Ecology 20:287-304.
Howe, H. F. and L. C. Westley. 1988. Ecological Relationships of Plants and Animals. Oxford University Press, New York, NY.
Laessle, A. M. 1942. T he plant communities of the Welaka Area. University of Florida Publications, Biological Sciences Series 4:5-141.
Landers, L. L. 1991. Disturbance influences on pine traits in the southeastern United States. Proceedings Tall Timbers Fire Ecology Conference 17:61-98.
Lemon, P.C. 1949. Successional responses of herbs in the longleaf-slash forest after fire. Ecology 30:135-145.
Marks, P. L. and P. A. Harcombe. 1981. Forest vegetation of the BIg Thicket, southeast Texas. Ecological Monographs 51:287-305.
Menges, E. S., W. G. Abrahamson, K. T. Givens, N. P. Gallo, and J. N. Layne. 1993. Twenty years of vegetation change in five long-unburned Florida plant communities. Journal of Vegetation Science 4:375-386.
Monk, C. D. 1968. Successional and environmental relationships of the forest vegetation of north central Florida. American Midland Naturalist 79:441-457.
Mutch, R. W. 1970. Wildland fires and ecosystems--a hypothesis. Ecology 51:1046-1051.
Myers, R. L. 1985. Fire and the dynamic relationship between Florida sandhill and sand pine scrub vegetation. Bulletin Torrey Botanical Club 112:241-252.
Peroni, P. A. and W .G. Abrahamson. 1986. Succession in Florida sandridge vegetation: a retrospective study. Florida Scientist 49:176-191.
Platt, W. J. and T. W. Doyle. 1994. Age structure of sand pine (Pinus clausa) stands in pennisular and panhandle Florida In preparation.
Quarterman, E. and K. Keever. 1962. Southern mixed hardwood forest: climax in the southeastern coastal plain: U.S.A. Ecological Monographs 32:167-185.
Rebertus, A. J., G. B. Williamson, and E. B. Moser. 1989. Longleaf pine pyrogenicity and turkey oak mortality in Florida xeric sandhills. Ecology 70:60-70.
Schwartz, M. W. 1994. Natural distribution and abundance of forest species and communities in northern Florida. Ecology 75:687-705.
Stout, I. J. and W. R. Marion 1993. Pine flatwoods and xeric pine forests of the southern (lower) coastal plain. Pages 373-446, In: W.H. Martin, S.G. Boyce, A.C. Echternacht, eds. Biodiversity of the Southeastern United States. Lowland Terrestrial Communities. Wiley & Sons, New York, New York.
Streng, D. R., J. S. Glitzenstein, and W. J. Platt. 1994. Evaluating season of burn in longleaf pine forests: a critical literature review and some results from an ongoing long-term study. Proceedings Tall Timbers Fire Ecology Conference (in press).
Tansley, A. G. 1935. The use and abuse of vegetational concepts and terms. Ecology 16:284-307.
Veno, P. A. 1976. Successional relationships of five Florida plant communities. Ecology 57:498-508.
Wahlenberg, W. G. 1946. Longleaf Pine: its use, ecology, regeneration, protection, growth and management. Charles Lathrop Pack Forestry Foundation, Washington D.C.
Ware, S., C. Frost, and P. D. Doerr. 1993. Southern mixed hardwood forest: the former longleaf pine forest. Pages 447-493, In: W.H. Martin, S.G. Boyce, A.C. Echternacht, eds. Biodiversity of the Southeastern United States. Lowland Terrestrial Communities. Wiley & Sons , New York, New York.
Wells, B. W. 1928. Plant communities of the coastal plain of North Carolina and their successional relations. Ecology 9:230-242.
Whittaker, R. H. 1953. A consideration of climax theory: the climax as population and pattern. Ecological Monographs 2
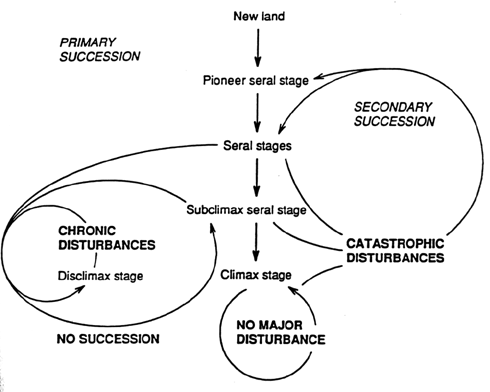
Figure 1. A diagrammatic representation of succession in terrestrial plant communities. Primary succession occurs following the formation of new land where the substrate has not been modified by vegetation. Pioneer stages are followed by successive seral stages in which the scale of disturbance decreases, until a an equilibrium climax community is present. Catastrophic disturbances during this transition from pioneer to climax stages reinitiate succession. Chronic, non-catastrophic disturbances can result in a persistent subclimax stage or cause a shift towards a disclimax stage.
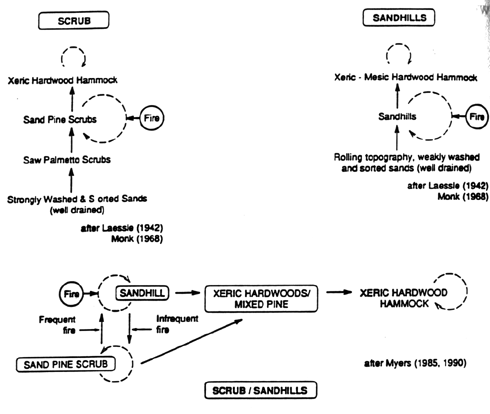
Figure 2. Successional models for Florida scrub (upper left), sandhills (upper right), and for both communities (lower). In the models of Laessle (1942, 1958) and Monk (1968), substrate differences are responsible for differences in the successional path of scrub and sandhills. In the models of Myers (1985, 1990), fire frequency is the primary cause of differences in the successional path of scrub and sandhills. All models predict convergence on hardwood hammocks if fire is excluded.
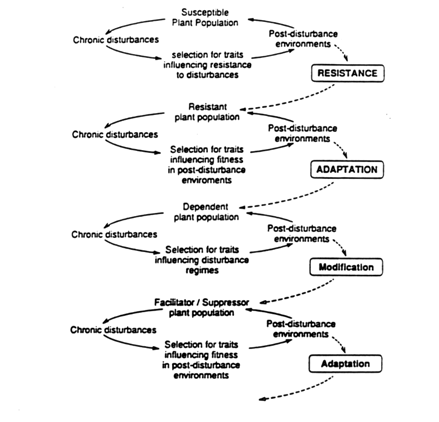
Figure 3. A three stage model for the evolution of plant populations exposed to chronic, noncatastrophic disturbances. If susceptible plant populations are exposed to such disturbances, traits that confer resistance are predicted to increase fitness. Once resistance occurs, traits influencing fitness in the post-disturbance environment should be subject to selection. Adaptation to post-disturbance environments is predicted to result in dependence on disturbance. Traits that influence the disturbance regime, either as facilitation or suppression of characteristics of disturbances, also are predicted to be subject to selection. Any modification of the disturbance regime should impose new selection pressures on indigenous plant populations, possibly resulting in adaptation to altered disturbance regimes. As a result of continual changes in the plant populations and the disturbance regime, no equilibrium is reached. This model is predicated on disturbances being chronic and noncatastrophic in nature over periods of time long enough for adaptation to occur. Limits to "coevolution" presumably are set by genetic variation in plant populations and variability in the disturbance regime.