Standard Operating Procedures for the Health Effects Division FQPA Safety Factor Meeting
Standard Operating Procedures for the Health Effects Division
FQPA Safety Factor Committee
You will need Adobe Reader to view some of the files on this page. See EPA's PDF page to learn more.
April 26, 1999
I. BACKGROUND
The Food Quality Protection Act of 1996 (FQPA) requires that in the case of threshold effects "an additional tenfold margin of safety for the pesticide chemical residue and other sources of exposure be applied to infants and children to take into account potential pre- and post-natal toxicity and completeness of the data with respect to exposure and toxicity to infants and children......the Administrator may use a different margin of safety for the pesticide chemical residue only if, on the basis of reliable data, such margin will be safe for infants and children". The FQPA Safety Factor Committee will make FQPA safety factor recommendations on all food-use chemical risk assessments generated by OPP following the guidance in the policy paper, The Office of Pesticide Programs' Policy on Determination of the Appropriate FQPA Safety Factor(s) for Use in the Tolerance-Setting Process (149 KB, PDF).
II. SCOPE
In determining whether an additional FQPA safety factor is needed, the Health Effects Division (HED) Safety Factor Committee (FQPA SFC) will consider: 1) the contribution of hazard and dose response evaluations; 2) the contribution of exposure assessment(s); 3) the characterization of the hazard (toxicology data base) and exposure (dietary food, dietary drinking water, and residential) data bases; and 4) the degree of concern regarding the potential for pre- and postnatal effects. Each pesticide will be considered on a case-by-case basis and the safety factor recommendation will be made using a weight-of-evidence approach in the course of the risk assessment process as the risk characterization is being developed and the hazard and exposure assessments are being completed.
III. PROCEDURES
(For special procedures for Section 18 Emergency Exemptions,
see Section VIII below.)
In order to assess the completeness of the data used in risk assessments and any potential effects on infants and children, the Committee will have at its disposal the report of the HED Hazard Identification Assessment Review Committee (HIARC) and written responses to sets of standard questions regarding the hazard and exposure information (dietary food, dietary drinking water and residential exposure considerations) prepared by the reviewers.
Using the guidance provided in the Office of Pesticide Programs' Policy document, the final FQPA safety factor recommendation is made by consensus of the Committee members based upon weight-of-evidence consideration of: 1) the level of confidence in the hazard and exposure assessments; 2) the degree of concern for potential toxicity to the unborn, infants and children; and 3) any residual uncertainties that are not accounted for in the hazard and exposure assessments. If a consensus cannot be reached on the safety factor recommendation, the Committee will seek guidance from the HED, Environmental Fate and Effects Division (EFED), Registration Division (RD), and Special Review and Reregistration Division (SRRD) Directors. The final decision on the FQPA safety factor is made by OPP senior management, informed by the science presented in the risk characterization and the recommendation of the HED FQPA Safety Factor Committee.
IV. ATTENDEES
All chemical reviewers, risk assessors, designated branch chiefs, and appropriate risk managers representing RD or SRRD will attend the meeting to answer any additional questions that may arise during the deliberations of the committee. Each of the hazard and exposure assessment areas should be represented (toxicology, dietary food exposure, dietary drinking water exposure, and residential exposure). If a reviewer, assessor, or risk manager is unable to attend, that reviewer, assessor, or risk manager will designate an appropriate person to attend in their absence.
V. SCHEDULING A MEETING
(For Section 18 Emergency Exemptions, see Section
VIII below.)
The FQPA Safety Factor Committee (FQPA SFC) will work in conjunction with the HIARC in reviewing registration and reregistration chemicals (FQPA SFC meeting will be scheduled approximately four weeks after HIARC) and the schedule for the FQPA SFC meetings will be incorporated into the master SARC schedule on the LAN (T:\HED\SCHEDULE).
For those chemicals not currently listed on the master SARC schedule, the lead reviewer for the chemical risk assessment will contact the Executive Secretary and/or Committee Chair to schedule a meeting date.
VI. STANDARD INFORMATION TO BE PROVIDED BY REVIEWERS
The FQPA safety factor recommendation will be based in part on the answers to the following questions. The Committee seeks characterization of the uncertainties in the data base used for the hazard and exposure assessments, as well as, of the susceptibilities of infants and children.
i. Toxicological Considerations for FQPA Safety Factor SelectionYour scientific judgment and qualitative description are just as important as quantitative data in characterizing the hazard in terms of the reliability of data, sources of uncertainty, severity of the effects, and the likelihood of effects on infants and children. Keep in mind the goal of these questions (i.e., characterization of hazard). Reference to the final HIARC report is adequate, provided that the information contained in the report completely addresses the question(s).
- Has the scientific quality of the toxicology data base and the confidence in the hazard endpoints and dose-response assessments been completely characterized?
- Do we have adequate hazard studies for evaluation of risk to infants and children? These include, but are not limited to, prenatal developmental toxicity studies in rodents and nonrodents; two-generation reproduction study in rodents; acute and subchronic neurotoxicity studies in rats, and developmental neurotoxicity study in rodents. Are additional studies being requested which have been triggered by the existing studies?
- Do these studies show quantitative increased susceptibility to infants and children? That is, do the effects in the young occur at doses not causing effects in the adults (describe the relative potency of the response)?
- Do these studies show qualitative increased susceptibility to infants and children? That is, are the effects in the young at the same level but more severe than those observed in the adults?
- Do effects occur in more than one species? If so, are they the same type of effects? Are the effects consistent or repeatable across species, generations, or studies?
- Are the observed effects dose-related, that is, does the incidence and intensity of response increase with increasing dose? Completely describe the spectrum of effects in both adult and young animals (include a characterization of the dose response curve, the latency and reversibility of effects, if known, etc.).
- If there is an increased concern for the health of infants and children, to what extent was this accounted for in identifying hazard endpoints (in the HIARC toxicology endpoint selection process) and in deriving hazard values for this pesticide (e.g., calculation of acute or chronic RfDs)?
- Provide a complete description of the uncertainty and/or modifying factor(s) used in calculating hazard values for this pesticide. NOTE: Reference to the final HIARC report is adequate, provided that the rationale for the factor(s) required is complete.
- Describe available mechanism of action studies which may provide information on precursor effects at lower doses. Are there comparative metabolism and/or pharmacokinetic (toxicokinetic) studies evaluating the dose at the target site and/or the duration of effect? If so, describe the findings of these studies.
- Describe any other studies (e.g., from the published literature) that are available for consideration which might influence a FQPA safety factor finding.
ii. Dietary Food Exposure Considerations for FQPA Safety Factor Selection
Your scientific judgment and qualitative description are just as important as quantitative data in characterizing the exposure in terms of the reliability of data, sources of uncertainty, magnitude of the exposure (is the actual exposure level being overestimated or underestimated), and identification of the populations exposed. Keep in mind the goal of these questions (i.e., characterization of the exposure).
- What is the likelihood of quantifiable residues in food? Describe (semi-quantitatively - there is no need to reproduce the labels) the typical use rates and frequency of application.
- Are there established tolerances? If so, cite 40CFR. What metabolites require regulation? Is there likelihood of transfer of residues to meat and/or milk? Are there Codex MRL's for the compound? If so, on what commodities?
- Are the residues systemic (taken up by and distributed throughout the plant)? Are residues likely to be removed during routine preparation such as washing, peeling, cooking, etc.? From the residue decline data, semi-quantitatively estimate how quickly this pesticide will dissipate (i.e., half-life).
- State and characterize the available residue databases for each crop (e.g., field study data, sources of available monitoring data - PDP, FDA, etc.). What are the limits of quantitation used? Describe semi-quantitatively the results of residue testing (range and frequency of positive findings, etc.).
- Is there information available on % crop treated? If so, identify the source of the information and the uncertainties around the number. What is the likely maximum % crop treated for each crop (based on potential market)?
- Is this pesticide used on foods considered to be highly consumed by infants and children? Based on the consumption data base used by DEEM, which crops contribute significantly to the diet for adults? Which contribute significantly to the diet of infants and children? Describe the degree of refinement (Tier) of the DEEM analyses for acute and chronic exposure.
iii. Dietary Drinking Water Exposure Considerations for FQPA Safety Factor Selection
Your scientific judgment and qualitative description are just as important as quantitative data in characterizing the exposure in terms of the reliability of data, sources of uncertainty, magnitude of the exposure (is the actual exposure level being overestimated or underestimated), and identification of the populations exposed. Keep in mind the goal of these questions (i.e., characterization of the exposure).
- Is the environmental fate database complete enough to characterize drinking water exposure?
a) Provide a brief summary of the environmental fate assessment for this compound and any metabolite(s) that may potentially get into drinking water based on metabolite fate characteristics.
b) Is the compound or any of its metabolites mobile and/or persistent? (A bottom line summary statement on drinking water exposure potential should be included.)- Discuss method for drinking water exposure assessment (e.g., monitoring data, modeling, combination).
a) If models are used, discuss which models, describe the resulting estimated environmental concentrations (EECs), the scenarios used in the model, and the degree of confidence in the modeling output.
b) If monitoring data are used (ground water or surface water), describe the monitoring data and conditions under which they were collected (e.g., from vulnerable areas at maximum label rates). Characterize the relationship between the monitoring data and the actual population exposed to this drinking water contaminant.- Please discuss the magnitude of the population potentially exposed to the pesticide via drinking water based on the extent of usage and whether the chemical characteristics indicate a likelihood of drinking water contamination.
iv. Residential Exposure Considerations for FQPA Safety Factor Selection
Your scientific judgment and qualitative description are just as important as quantitative data in characterizing the exposure in terms of the reliability of data, sources of uncertainty, magnitude of the exposure (is the actual exposure level being overestimated or underestimated), and identification of the populations exposed. Keep in mind the goal of these questions (i.e., characterization of the exposure).
- Is the compound used in such a way that infants and/or children may be exposed? If so, describe the specific residential exposure scenarios of concern for infants and children. What is the frequency and magnitude of the use?
- Have Pesticide Handler Exposure Database (PHED) data been used in estimating the exposure? How well does the PHED scenario reflect the actual use pattern? Rate the data used based on the PHED grading criteria (high quality, medium quality, or low quality). If chemical-specific or other non-PHED data have been used, describe the scope of the study, resulting exposure values, and general quality of the study.
- Have the Draft Standard Operating Procedures for Residential Exposure Assessments been used as the basis for estimating the exposure? Describe any deviations from DRAFT SOP calculations and the impact on the assessment results (e.g., assessment reflects a less conservative approach by altering transfer coefficient value for dermal exposure).
- Is chemical-specific product use information available through BEAD or some other source? Has the assessment been developed to reflect this information? Has this information been used as a basis for characterizing the populations considered in the assessment?
- Are reliable biologically-based exposure data or epidemiology data available to support the results of the assessment (e.g., incident report, CDC biomonitoring data, etc.)?
- Have models other than PHED or those presented in the DRAFT Residential SOPs been used to calculate dose in any aspect of the assessment (e.g., CONSEXPO, TherDbase, etc)? Summarize how these are integrated into the assessment.
- Is 100% dermal absorption assumed (when dermal endpoints are derived from oral studies)? Or, are there chemical-specific data available that indicate a different dermal absorption rate?
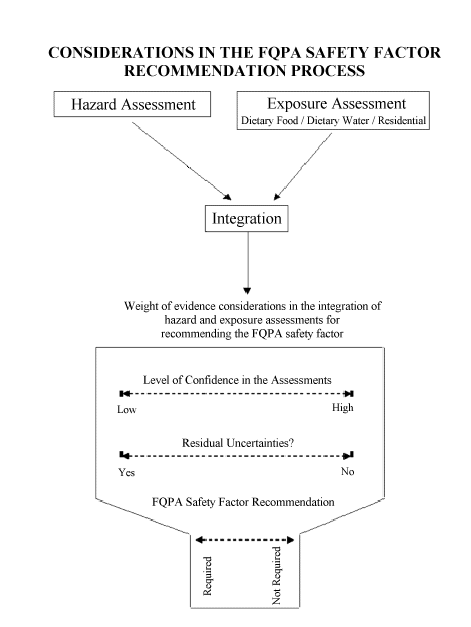
VII. ASSESSMENT INTEGRATION AND RECOMMENDATION REPORTING
The integration task assigned to the Committee is to characterize the overall confidence that infants and children would be protected using the weight-of-evidence considerations illustrated in the figure below. These considerations include the level of confidence in the hazard and the exposure assessments, the characterization of the degree of concern, and whether there are residual uncertainties that are not accounted for in these assessments.
The final report of the FQPA SFC will include: 1) a summary of the hazard and exposure assessments presented at the meeting; 2) characterization of the integrated assessment, including the degree of concern; 3) the FQPA safety factor recommendation based on the weight-of-evidence considerations and the rationale used to select it; and 4) designation of the population subgroups for which the safety factor is applicable.
VIII. TIERED APPROACH TO FQPA SAFETY FACTOR RECOMMENDATIONS FOR EXPEDITED ACTIONS
- A "Tier I" risk assessment for expedited actions (i.e., Section
18 exemptions) is performed, assuming that a safety factor of
10x (as required by FQPA) is retained. If risk estimates do not
exceed HED's level of concern under these circumstances, the action
goes forward noting that the safety factor determination applies
only to this action and is subject to change when the chemical
undergoes full review by the FQPA Safety Factor Committee.
- If risk estimates exceed HED's level of concern when a safety factor of 10x is assumed to be retained, the Section 18 team should refer to the HED Toxicology Endpoint Selection and FQPA Process for Expedited Actions (March 22,1999).
![[logo] US EPA](../gif/logo_epaseal.gif)