February 1999, Aldicarb Evaluation
Evaluation and Incorporation of the Time for Reversibility of
the Adverse Effects of Aldicarb
(1/22/99 Draft)
Introduction | Background
| Toxicology Data | Exposure
Considerations
Framework for Decision-Making | References
| Questions
Introduction
The Office of Pesticide Programs (OPP) currently evaluates dietary exposure to pesticides in two broad time frames. A chronic exposure is one that occurs for a substantial portion of an individuals life. An acute exposure is one that lasts for approximately one day. The advent of calendar based models for estimating exposure has introduced the potential for using a sliding scale for time of exposure. Calendar based models attempt to model the time course and frequency of exposure events such that exposure and accompanying risk from a pesticide are consistent with likely use patterns. The major limitation for estimating exposure in detail is the scarcity of adequate data, including hazard and exposure data. These data are necessary to support an alternate time frame that attempt to focus the risk assessment process to finer time increments. This paper presents the data available and OPP's evaluation of the data to determine the acceptability of an alternate exposure time for the conduct of a dietary risk assessment for the pesticide aldicarb. The focus of this discussion is not the outcome of the risk assessment, but rather the evaluation process for determining the approach to application of the data. OPP is seeking the Panel's review of the decision making process by which the determination was made that an the risk assessment for aldicarb could incorporate and 8 hour period of reversibility in time course decisions.
Background
Aldicarb is a carbamate pesticide that exerts its pesticidal activity and elicits adverse toxic effects by inhibition of cholinesterase activity (ChEI). The acute dietary exposure to aldicarb has historically been evaluated using OPPs acute dietary risk assessment process that assumes that a daily exposure is the appropriate time frame for a risk assessment.
This approach has resulted in dietary risk assessments indicating that an unacceptable risk concern exists for consumption of foods treated with aldicarb. In order to better define the potential for risk from dietary exposure to aldicarb, the registrant Rhone-Poulenc Ag Company (RPAC) proposed the use of a dietary assessment using a shorter time frame. They argued that the shorter time frame was more reflective of the actual duration of the effects of aldicarb, and therefore provides a better representation of the risk picture. This position assumes that the duration of ChEI is a good surrogate for all potential adverse effects of aldicarb. OPP evaluated the toxicology data and exposure profile as outlined below.
Toxicology Data
The toxicology portion of this presentation is based on a memorandum (Sette, W to Housenger J 7/14/97). The memorandum was written to address the following questions:
- What period of time should be regarded as the duration of effects from an acute exposure to aldicarb?
- What interval of time is appropriate to use to define an acute consumption episode for commodities treated with aldicarb?
The text of the memo has been revised and extended for this meeting to improve the clarity of the analysis. Additional materials are taken from available studies and reviews that were part of the internal review on this set of issues. Where appropriate, these materials have also been revised and updated. Based on this analysis of all of the available data, the memo, concluded that 8 hours is a reasonable best estimate of the duration of aldicarb's effects and so, also, the acute eating interval. The rationale for this conclusion is presented below.
Rationale
A number of human studies and case series have provided information describing the effects of acute exposures to aldicarb in terms of the duration of clinical signs and symptoms and the time course of ChEI in blood. There are also animal studies of acute and repeated daily exposures that provide measures of clinical signs, other behavioral effects, and blood and brain ChEI. However, EPA knows of no systematic data on multiple, within-day exposures and differences in sensitivity in terms of these effects. Thus, the present analysis assumes that if observed behavioral or neurochemical effects are fully recovered, then sensitivity has returned to baseline. That is, the effects of exposure to aldicarb are assumed not to be cumulative within the 24 hour time frame, and recovery of ChEI and disappearance of clinical signs will be taken as an indication that the exposed animal or human subject would react to an additional exposure as if they had not been previously exposed.
Summary of Human Studies
A number of the critical controlled human studies and case series provide relevant data on the duration of illness and on the time course of ChEI reversibility.
Duration of clinical signs and symptoms
In RPAC's 1992 study, clinical signs and symptoms in men exposed to single doses of 0.025-0.075 mg aldicarb/kg body weight were reported to have a duration of 6 hours or less. These observations are supported by another controlled human study (Union Carbide, 1971) in which clinical signs and symptoms in groups of 4 men exposed to 0.025 - 0.1 mg aldicarb/kg body weight were also reported to have been resolved within 6 hours of exposure.
Some of the case series reported discuss the illness durations only in general terms, noting that they were generally short term, with quick resolution, though some are described as more serious and longer lasting. (Goldman et al. 1990 a,b). In other case series the duration of illness following inadvertent dietary exposures to aldicarb residues, effects appeared to persist beyond 8 hours. Goes et al. (1980) reported that 2 of 14 cases exhibited illnesses lasting 12 hours. Another, an 80 year old woman, was hospitalized for 24-36 hours, but the time of cessation of her illness was not noted. Green et al. (1987) indicated that the original family affected in their report recovered in 4 hours. They also reported that for a few individuals, sudden onset of effects occured only after a second ingestion. They discussed a physician who ate watermelon containing 0.01 ppm (limit of detection) 3 times, and who complained after the 1st portion that he "just didn't feel right"; after 18 hours, he ate a 2nd portion and noted that he felt dizzy & cloudy headed; and 5 hours later, he ate a 3rd portion and experienced diarrhea, nausea, disequilibrium, blurred vision, and an inability to think clearly. Hirsch et al. (1987), however, noted recovery of exposed individuals generally within 8 hours.
Duration of Cholinesterase (ChE) Inhibition
With respect to cholinesterase inhibition, RPAC's human study from 1992, which is the best available data set, showed statistically significant blood cholinesterase inhibition generally lasting 1-6 hours. Four figures from the study report show the effects on plasma and RBC ChE levels in men and women respectively (Figures 18.7-18.10). They all show the general pattern of effects, with dose dependent peak effects at one hour after dosing with a steady decline, generally returning to within 5-10% of baseline levels by 8 hours after exposure. Tables 18.2.1-18.2.4 from the study report, show the results of the statistical analyses which compared the mean value for each time after dosing minus the predose value to the mean difference for the placebo exposures at that time point.
A summary table based on these analyses shows the testing times at which each dose was found to be statistically significantly decreased in exposed men and women (Table 1).
Table 1: Statistically significant (p < 0.05)1 times after exposure for men (M) and women (F) exposed to acute oral doses of aldicarb.
| Time after Dosing | 1 HR | 2 HRS | 4 HRS | 6 HRS | 8 HRS | 21 HRS |
| Dose of Aldicarb | ||||||
| 0.01 mg/kg | ||||||
| M RBCS | X | X | X | X | ||
| M PLASMA | X | X | ||||
| 0.025 mg/kg | ||||||
| M RBCS | X | X | X | X | ||
| M PLASMA | X | X | X | X | ||
| F RBCS | X | X | ||||
| F PLASMA | X | X | X | |||
| 0.05 mg/kg | ||||||
| M RBCS | X | X | X | X | ||
| M PLASMA | X | X | X | X | X | X |
| F RBCS | X | X | X | |||
| F PLASMA | X | X | X | |||
| 0.075 mg/kg | ||||||
| M RBCS | X | X | X | X | ||
| M PLASMA | X | X | X | X | X | X |
- From this table one can see that between 1-2 hours after exposure, both men and women showed statistically significant decreases in both plasma and RBC ChEI at all doses tested, between 0.01 and 0.075 mg/kg.
- At 4 hours after exposures, men showed somewhat greater sensitivity than women, where for females at 0.025 mg/kg, only plasma was affected, while for men there were significant decreases in both plasma and RBCs at 0.025 mg/kg and in RBCs at 0.01mg/kg.
- At 6 hours after exposure, while the magnitude of effects were largely recovered, males were still affected, with plasma ChEI significantly decreased for 0.025-0.075 mg/kg (10-20%); while RBC ChEI was significantly affected only for 0.5 and 0.75 mg/kg (5-10%) (See Figures).
- Thereafter, at 8 and 21 hours (with two exceptions*), only the plasma ChE in men remained significantly decreased and only at the two highest doses. The level of inhibition at these times was quite small, 10% or less.
It should be noted, that EPA expressed some concerns in our review of that study regarding the red blood cell measures as potentially underestimated by incomplete lysing of the red blood cells (leaving an incomplete red blood cell quantity in the supernatant for analysis). Thus, the RBC values may underestimate the degree of inhibition.
In summary, for the ChEI data in this study, plasma and RBC ChEI were completely recovered by 8 hours after exposure for doses between 0.01 and 0.025 mg/kg in men and 0.025-0.05 mg/kg in women. At 8 and 21 hours, effects on plasma ChEI in men at 0.05 and 0.075 mg/kg persisted, but the inhibition was 10% or less, approaching a threshold effect level.
In the other controlled study (Union Carbide, 1971), whole blood ChE was reported to have largely, but perhaps not completely recovered by 6 hours after exposure (last period tested) of four men/dose to 0.025 - 0.1 mg/kg. At 6 hours after dosing, the range of inhibition for the 0.1 mg/kg group was between 35% inhibition and 44% above baseline; for 0.05 mg/kg, 0-16% inhibition, and 0.025 mg/kg, 3-24% inhibition.
Animal Data
The data supporting the chronic risk assessments for aldicarb and alidcarb sulfoxide are described in reports of the EPA Integrated Risk Information System (IRIS) (US EPA, 1993a,b). These sources summarize a variety of the long term exposure studies on these chemicals and the reference doses for chronic exposures.
There are also newer acute oral neurotoxicity studies of aldicarb, aldicarb sulfoxide, and aldicarb sulfone in rats which contain evaluation of clinical signs, other behavioral effects, and cholinesterase inhibition in brain and blood. The acute neurotoxicity studies in rats done according to the EPA guideline 81-7, (now 870.6200) focus on the time of peak effect on the day of dosing and one and 2 weeks post dosing to evaluate behavioral and neurochemical (ChEI) effects. Thus, they do not describe the complete time course of these effects between the time of peak effect and 7 days after exposure. Special studies of aldicarb and its 2 metabolites were requested to address the time course of ChE effects. This special study looked at clinical signs, and blood and brain ChEI prior to and 1,4, and 8 hours after exposure to each of these substances following acute exposure at doses that were considerable portions i.e., 25% or more the oral LD50. An additional study of aldicarb sulfone examined effects 24 and 48 hours after exposure. These studies utilized doses of these materials that were close to lethal levels.
In the special acute neurotoxicity studies of Aldicarb, Aldicarb Sulfoxide, and Aldicarb Sulfone (MRID 43442305) groups of 18 Sprague-Dawley rats received acute oral doses by gavage of: aldicarb or aldicarb sulfoxide at doses of 0.25 or 0.5 mg/kg (vol 2 ml/kg); and aldicarb sulfone at doses of 10 and 20 mg/kg (vol 10 ml/kg). A control group received water at a volume of 10 ml/kg. Body weights and clinical observations were made prior to exposure and prior to sacrifice on 6/sex/dose. Blood samples were taken 1,4,or 8 hours after dosing on 6/sex/dose from the abdominal aorta under anesthesia. Left hemisphere and right frontal cortex, hippocampus, cerebellum and caudate/putamen were weighed and ChEs analyzed.
There were no deaths or differences in body or brain weights. Clinical signs were seen in all dose groups for all 3 chemicals 1 hour after dosing and included: tremors, salivation and peri-orbital, muzzle, abdominal and urogenital staining or wetness. 8 hours after dosing, these signs were still evident in some rats given aldicarb sulfone(tremors in 5/6 males), but were only incidentally seen in aldicarb rats (muzzle and peri-orbital staining in 1/6 rats), and were no longer seen in rats given aldicarb sulfoxide.
Statistically significant and large reductions in brain (> 20%) and blood cholinesterases (> 66%) were noted in most groups 1 hour after treatment. At 4 hours after dosing, for 0.25 mg/kg of aldicarb and sulfoxide, inhibition had generally recovered (< 4% brain;< 24% blood ; at 8 hours for 0.5 mg/kg of either chemical, inhibition had also generally recovered (< 3 %, brain; < 27 % blood. But for aldicarb sulfone, effects were still apparent after 8 hours at both doses (46- 57 % brain; 51-63 % blood). Based on the persistence of the effects of aldicarb sulfone on behavior, brain and blood ChEs, another study was conducted to examine 5 rats/sex/dose 1, 8, and 24 or 48 hours after exposure to 10 and 20 mg/kg. In both sexes 24 hours after exposure, plasma ChEI was still significantly decreased (50%) at 20 mg/kg. Brain ChEI was still significantly decreased (29-35% inhibition, whole brain). 1/5 females showed some staining. By 48 hours after exposure, ChEI recovery was seen in all compartments, and 1/5 female rats still exhibited some staining.
While clearly the duration of effects are dose dependent, and this long duration of effect would not be expected at lower doses, the data suggest that the duration of effects of sulfone may be much longer lasting than those of aldicarb or aldicarb sulfoxide.
The acute rat neurotoxicity study of aldicarb (MRID 43442301) showed an LOAEL of 0.05 mg/kg for plasma cholinesterase inhibition (33%, males - 47%, females) at the time of peak effect, 0.75 hours after the exposure, and an LOAEL for clinical signs of 0.1 mg/kg (for decreased forelimb grip strength, females). The time course of these effects were not measured in this study.
LOAELs and NOAELs in rats and humans following acute exposures show some overlap, though humans appear slightly more sensitive. The NOAEL in humans for both clinical signs and ChE inhibition is 0.01 mg/kg. In the acute rat neurotoxicity study, the NOAEL for clinical signs was 0.5 mg/kg, and ChE inhibition was only determined to be less than 0.05 mg/kg, the lowest dose tested. Humans and rats also show similar levels of whole blood or plasma ChE inhibition following acute exposures, though the ranges are quite broad which somewhat limits the precision of the comparisons. Table 2 compares effects seen in humans and rats after acute exposures at several dose levels.
Table 2: Comparison of acute rat and human data
| Dose (mg/kg) | Rats | Humans |
| ChEI 0.1 mg/kg | 54-61%, whole blood | 32-80%, whole blood, UC |
| ChEI 0.05 mg/kg | 33-47%, plasma | 55-68%, plasma, RPAC |
| Signs 0.1 mg/kg | LOAEL, forelimb grip strength | 4/4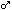 ; sweating,
leg weakness, miosis, other effects. UC ; sweating,
leg weakness, miosis, other effects. UC |
| 0.05 mg/kg | NOAEL, signs | 1/8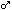 ; sweating
RPAC ; sweating
RPAC |
| 0.025 mg/kg | LOAEL, sweating 1/8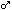 ;
RPAC ;
RPAC |
|
| 0.010 mg/kg | NOAEL, signs and ChEI |
The NOAEL for clinical signs is somewhat lower for humans (men), as is the peak level of inhibition seen at 0.05 or 0.1 mg/kg in blood.
There are also repeated exposure studies in animals of aldicarb which provide little indication of any cumulative toxicity resulting from multiple exposures across weeks or months. By and large, these repeated exposure animal studies do not show cumulative toxicity from aldicarb. The NOAELs from subchronic exposures are similar to those from acute exposures in terms of overt effects or in terms of cholinesterase inhibition. The current rationale for the reference dose (US EPA, 1993a) notes: " Based on a relatively complete data base for systemic toxicity, [clinical signs] effects from repeated exposures have not been seen in animals at levels comparable to those seen in humans exposed acutely, and so would not support a lower RfD.
While we lack any human data on the adverse consequences of repeated aldicarb exposure, available evidence with aldicarb both in experimental animals and in humans suggest that neuro-behavioral effects are short lived with essentially no indication of accumulation of effects over time. Thus, the doses producing effects following repeated daily exposure are comparable to those following a single dose. Using inhibition of cholinesterase activity as a bio-marker of exposure, one notes comparable degrees of inhibition from essentially the same dose whether delivered once or following subchronic or chronic dosing (See e.g., RPAC, 1988; RPAC, 1992 2). Since aldicarb appears not to produce neuro-behavioral effects at doses equivalent or below those producing inhibition of cholinesterase, an acute human experimental study is expected to reasonably evaluate the potential neuro-behavioral consequences of repeated human exposure."
Conclusions
- For the controlled studies and in most of the case reports in humans, effects of acute exposures were resolved within 6 hours of exposure.
- For cholinesterase inhibition measured in the controlled studies, there were significant changes in blood ChEI 6 hours after exposure with resolution in general by 8 hours after exposure, particularly at lower doses.
- Both behavioral effects and ChE inhibition in animals exposed acutely to aldicarb, aldicarb sulfoxide, or aldicarb sulfone also showed general recovery of both clinical signs and ChEI within 8 hours following acute exposures. Effects of aldicarb sulfone were still apparent after 8-24 hours later , but only at doses close to lethal levels.
- Effect levels for rats, dogs, and humans seemed broadly comparable for ChEI in acute studies, although humans seemed slightly more sensitive to effects of acute exposure.
- Animal data showed little evidence of cumulative toxicity across subchronic or even chronic exposures, implying that multiple exposures in an acute framework might not pose an additional concern for cumulative effects.
In conclusion, an interval of 8 hours was selected as a reasonable estimate of an interval during which adverse overt clinical effects and ChEI would be recovered.
Exposure Considerations
Based upon the decision that the effects of aldicarb were reversed within 8 hours of the discontinuation of exposure, a modification was proposed by RPAC to OPP's standard approach to acute dietary risk assessment. The dietary assessment submitted by RPAC was conducted using the DEEM software developed by Novigen Sciences, Inc. The software uses the consumption records of individuals from the Continuing Survey of Food Intakes by Individuals (CSFII) 1989-1991 to calculate the potential exposure to an individual from commodities reported consumed during the survey. The consumption data are recorded as foods consumed throughout the course of three 24-hour periods. Standard OPP practice is to accumulate the consumption for each 24-hour period and use the consumption as representative of a daily exposure.
The RPAC dietary assessment proposed a more detailed use of the consumption records from the CSFII to permit the identification of "clear" periods during which the individual in question could be assumed to have returned to effectively baseline state. This would permit the introduction of "zero" value which is reflective of the modelled exposure for periods during which the individual was determined to be likely to be free of the effects of exposure of aldicarb and during which no additional exposure occurred. The initial proposal by RPAC was the use of a 6 hour period for recovery. OPP determined that an 8 hour period was more appropriate based upon the available data.
OPP expressed the concern that additional exposure to aldicarb before complete recovery from a previous exposure could result in effects for which no data were available. As indicated above, no data exist on the impact of multiple exposures prior to reversal of the effects of the previous exposure. To address this concern, the assessment was conducted with the requirement that a full 8 hours must have elapsed between consumption of a food with a tolerance and the presumption of return to baseline. In this way, the reversibility issue was addressed by preventing introduction of "zero" exposure values more frequently than was supported by the data.
To better demonstrate the evaluation of a consumption diary, the
following example is provided. Assume that tolerances exist for
a pesticide on potatoes, carrots and almonds. The two consumption
diaries might be used as follows:
| Diary #1 | Diary #2 | |
| 7am | Cereal with almonds Orange juice Milk |
Oatmeal Coffee with cream |
| 9am | Danish | |
| 12pm | Potato chips Corned beef sandwich Diet soda |
Vegetarian chili Corn chips Carrot juice |
| 3pm | Diet soda Cookies |
|
| 6pm | Steak Fried Potatoes Green beans Iced tea, sweetened |
Salad Roll Salad dressing Water |
| 9pm | Chocolate almond bar |
For diary #1, no eight hour period during the day can be identified during which there is no consumption of a food potentially treated with the pesticide. Assuming an eight hour effect window, the only clear period for the day would begin at 9 pm after consumption of the last food before retiring. On the other hand, diary #2 presents a case where only the carrot juice consumed at noon would have potential for exposure. The period prior to noon and from 8 pm on would be considered to be free of concern.
Framework for Decision-Making
During evaluation of the RPAC dietary exposure assessment OPP developed a series of criteria that had to be met to permit the inclusion of exposure free periods in the assessment. The criteria are for both exposure and for toxicology data and were used to outline the case for aldicarb and the adoption of this approach. In addition, OPP discussed the possible extension of this approach to other carbamate pesticides. The criteria for decision-making are presented below.
Toxicology Considerations
- Is the case for reversibility of the effects of aldicarb as
indicated by the toxicity data adequate to support use in a modified
risk assessment? (OPP determined "YES")
- Both behavioral and neurochemical effects are reversed within 8 hours of acute exposure.
- LOAELs from chronic exposures are comparable to or not much lower than LOAELs from acute exposures, and little, if any, cumulative toxicity is observed in the animal data.
- Effects seen in different species are consistent and occur at similar doses.
- The sulfone has longer lasting effects (8-24 hours) at very high doses in rats, but this is not expected at the lower doses where there are exposure concerns.
- What data are needed to make the case for reversibility and
alteration of time frame?
- Ideally, multiple within day exposures to establish whether there is any change in sensitivity
- Time course for clinical signs after acute exposures.
- Time course for cholinesterase inhibition after acute exposures.
- Comparison of exposures of different duration (e.g., chronic vs acute studies) and the differences in LOAELs and NOAELs observed to determine the extent of cumulative toxicity and the durations under which it occurs.
- Multi-dose comparisons to demonstrate how increasing dose impacts the period of reversibility.
Exposure Considerations
- Is the use of a period of less than 24 hours warranted for aldicarb?
(OPP answered "YES")
- The toxicology data have been found to support the argument that the effects are fully reversible, and the time course of reversibility is well defined.
- Consumption data from CSFII record consumption patterns throughout the day such that the occurrence of 8 hour periods with no consumption of potentially aldicarb treated foods can be identified.
- Data are available on the variability of levels of aldicarb in specific commodities of concern.
- What data are needed to support this assessment?
- Water exposure data (24 hour consumption data and residue data)
- Residue field trial data (typical vs maximum rates)
- Single serving data or estimates of variability in composites.
References
Goes EA, Savage EP, Gibbons G, Aaronson M, Ford SA, and Wheeler HW. 1980. Suspected Foodborne Carbamate Pesticide Intoxications Associated with Ingestion of Hydroponic Cucumbers. Amer J Epidemiol 111:2 254-260.
Goldman, LR, Beller M, and Jackson RJ. 1990. Aldicarb food poisonings in California, 1985-1988: Toxicity estimates for humans. Arch Environ Health. 45(3): 141-147. MRID 44699601.
Goldman LR, Smith DF, Neutra RR, Duncan Saunders L, Pond EM, Stratton J, Waller K, Jackson RJ, Kizer K. 1990. Pesticide Food Poisoning from Contaminated Watermelons in California, 1985. Arch Environ Health. 45(4) 229-236. MRID 44699602.
Green MA, Heumann MA, Wehr M, Foster LR, Williams pg, Polder JA, Morgan CL, Wagner SL, Wanke LW, and Witt JM. 1987. An Outbreak of Watermelon-Borne Pesticide Toxicity Amer J Pub Health 77: 11 1431-1434.
Hirsch G.H., Mori B.T., Morgan G.B., Bennett P.R., and Williams B.C. 1987. "Report of Illnesses Caused by Aldicarb-Contaminated Cucumbers." Food Additives and Contaminants. 5:2, pp. 155-60. MRID 44699603.
Rhône-Poulenc Ag Company (sponsor). 1992. "A Safety and Tolerability Study of Aldicarb at Various Dose Levels in Healthy Male and Female Volunteers." Inveresk Clinical Research Report No. 7786. MRID 42373001 HED Doc No 010459
Union Carbide Corporation. 1971. "Ingestion of Aldicarb by Human Volunteers: A Controlled Study of the Effects of Aldicarb on Man." Union Carbide Corporation Study No. ALD-03-77-2215. February 11, 1971. MRID No. 00101911; HED Doc. Nos. 007601, 010450.
US EPA, 1993a Aldicarb. US EPA IRIS Substance file.
https://www.epa.gov/iris/subst/0003.htm
US EPA, 1993b Aldicarb sulfone. US EPA IRIS Substance file.
https://www.epa.gov/iris/subst/0312.htm
Union Carbide Corporation. 1971. Haines R, Dernehl JB, Block JB, supervising physicians. Ingestion of Aldicarb by Human Volunteers: A Controlled Study of the Effects of Aldicarb on Man. ALD-03-77-2215. Feb 11, 1971. Available from EPA. Write to FOI, EPA, Washington, DC 20460. MRID 00101911 HED Doc. Nos. 07601, 010450
US EPA 1997. Memorandum dated 7/14/97 from W Sette to J Housenger entitled "Duration of Aldicarb's Effects.
1Based on a comparison of differences between means between placebo and dose groups, where the means were calculated as the differences between the post and pre dose value. Back to Table 1
2The chronic dog study (RPAC, 1988) had an LOAEL of 0.028 mg/kg/day, based on 21-28% plasma ChEI, but no effects on RBC ChEs or on clinical signs. In the 1992 RPAC acute human study, 0.025 mg/kg resulted in 35-49% plasma ChEI, 12-20% RBC ChEI, and on sweating in one man. Back to Text
Scientific Advisory Panel (SAP): February 1999 Meeting![[logo] US EPA](../gif/logo_epaseal.gif)