Release of Heavy Metals from Ironite®
Note: EPA no longer updates this information, but it may be useful as a reference or resource.
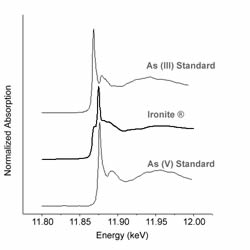
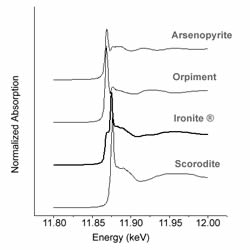
Ironite®, once available at many lawn and garden stores, was a common fertilizer made from mine tailings. The presence of heavy metals in Ironite® lead to its banning in Canada and lawsuits in the United States due to the potential release of heavy metals–notably arsenic and lead. Bioavailable arsenic released from Ironite® is dependent on its mineralogical form.
Earlier research sponsored by the producer of Ironite® identified the arsenic-bearing phase as arsenopyrite, with the conclusion that arsenic in that form does not pose an ecological threat. However, a closer look with Extended X-ray Absorption Fine Structure (EXAFS) has concluded the arsenic phase within Ironite® to be scorodite-like. Scorodite is more soluble than arsenopyrite. In fact, the dissolved arsenic released from scorodite can exceed the U.S. standards for drinking water. In addition to the data collected at Argonne National Labs in February 2005 that identified arsenate sorbed to iron oxides as the dominant arsenic bearing phase, secondary identification techniques are being used to confirm this finding such as thermogravimetric analysis and Mössbauer spectroscopy.
US DOE (2006) Argonne National Laborator APS Science 2006 Report. (ANL-06-23) (PDF)) "IRONITE: A Potentially Fertile Source of Soil Contamination.." p. 102-3
Contacts
Kirk Scheckel
513-487-2865
U.S. EPA National Risk Management Research Laboratory
Land Remediation and Pollution Control Division
26 W. Martin Luther King Dr.
Mail Code: CHL
Cincinnati, OH 45268
Christopher Impellitteri
513-487-2872
U.S. EPA National Risk Management Research Laboratory
Land Remediation and Pollution Control Division
26 W. Martin Luther King Dr.
Mail Code: 681
Cincinnati, OH 45268
Thabet Tolaymat
513-487-2860
U.S. EPA National Risk Management Research Laboratory
Land Remediation and Pollution Control Division
26 W. Martin Luther King Dr.
Mail Code: CHL
Cincinnati, OH 45268