Extramural Research
Presentation Abstract
Grantee Research Project Results
Advanced Nanosensors for Continuous Monitoring of Heavy Metals
Adam K. Wanekaya#, Daniel Andreescu#, Omowunmi
A. Sadik#,* Joseph Wang§*
#Chemistry Department, State University of New York at Binghamton, Binghamton NY 13902.
§*Department of Chemistry & Biochemistry, New Mexico State University, PO Box 30001, MSC 3C, Las Cruces, NM 88003-8001.
*Corresponding Author: osadik@binghamton.edu; joewang@nmsu.edu
Summary:
The overall objective of this work is to utilize novel colloidal-metal
nanoparticles that are incorporated into a bed of electrically conducting
polymers (ECPs) for the development of nanosensors. We explored the feasibility
of designing advanced conducting materials for sensor and remediation
applications. Specifically, we examined the synthesis of (i) polyamic
acid-silver nanoparticle composites membranes, (ii) polyoxy-dianiline
films and (iii) electrochemical deposition of gold nanoparticle films
on functionalized conducting polymer substrates. We present here a short
description of our fast and simple synthetic approach to gold nanoparticles.
This one-step, synthesis of stable gold nanoparticles uses polyamic acids
as both the reducing and stabilizing agents. The nanostructured materials
were characterized using electrochemical and morphological techniques
such as Fourier-transform infrared spectroscopy, cyclic voltammetry, galvanostatic
methods, Energy-dispersive spectroscopy, and transmission electron microscopy.
Novel gold nanoparticles were prepared through the reduction of AuCl3
by polyamic acid in organic medium in less than an hour. The polyamic
acid acts as a reducing agent of the metal salt and a stabilizing agent
of the resulting Au nanoparticles. The procedure resulted in gold nanoparticles
capped with the p-conjugated polyamic acid. Depending on the reactant
concentrations and ratios, the polyamic acid-metal hybrid were synthesized
either as well-dispersed or aggregated particles. The size of the particles
which can be controlled by varying the polyamic acid:AuCl3
ratios ranged from 4.0 ± 0.7 nm to 7.8 ± 1.0 nm. Potential
applications and environmental implications of the proposed materials
will also be discussed.
Introduction
Current widespread interest in metal nanomaterials is driven largely by
a large number of potential applications including environmental catalysts,
ultrafast optical switches, sensors and surface-enhanced spectroscopies.
Many of these applications require the nanoparticles to be stable and
evenly distributed with precise control of size, geometry and morphology.
The stable dispersion of nanoparticles in water is important to many applications.
However, the water-based synthesis of nanoparticles is fraught with inherent
problems such as ionic interaction, low reactant concentration, and difficulty
in removing the residue of stabilizers after synthesis. Particles synthesized
in organic solvents can be made at relatively high concentration with
predefined size and shape and with improved monodispersibility when compared
with those prepared in aqueous media. Most reports on the synthesis of
gold nanoparticles in non-polar organic solvents have followed the Brust
protocol2 wherein aqueous chloroaurate ions are transferred into the organic
solvent using phase-transfer molecules (tetraalkylammonium salts).
KBH4 or alcohol has been used for the reduction of the gold salt to form the nanoparticles before being stabilized and protected by the linear polymer. Other workers have since reported the stabilization of Au nanoparticles using linear polymers. Polymer molecules that possess functional groups such as SH, CN and NH2, can provide sites to bind with Au nanoparticles and prevent them from aggregating since those functional groups are known to have a high affinity for Au. Poly(dithiafulvene) represents the first example where a polymer was used both as a reducing agent and a stabilizing agent in the preparation of gold nanoparticles in organic medium. However, the reaction with poly(dithiafulvene) took 24 hours.
In this work, we report what is to our knowledge the first report on
the synthesis and stabilization of gold nanoparticles using polyamic acid
both as a reducing and stabilizing agent in the solution phase. This approach
is based on the reduction of gold III chloride by the polyamic acid and
its subsequent capping and stabilization of the resulting gold nanoparticles.
Reduction of AuCl3 by this  -conjugated
polymer at room temperature led to gold nanoparticles with narrow size
distribution and high dispersity with the resulting oxidized conjugated
polymers protecting the gold nanoparticles as stable colloidal solution.
The size of the nanoparticles was controlled by simple variation in the
polyamic acid:AuCl3 molar ratio. Unlike poly(amidoamine) dendrimer-encapsulated
gold nanoparticles that are only stable for 1 day before sedimentation,
the current synthesis and stabilization with polyamic acid allows stability
for several months with no change in dispersion, nanoparticle density,
size distribution or absorption spectra when stored at –20º
C. Furthermore, the polyamic acid-capped gold nanoparticles are formed
in less than 1 hour in a rapid, one-step, single-phase synthesis compared
to those produced in 12 hours to 1 day.11 Also, some current
approaches are performed in two phases and thus require extraction into
the organic medium.The PAA reaction enables the direct formation and stabilization
of the nanoparticles in the organic medium.
-conjugated
polymer at room temperature led to gold nanoparticles with narrow size
distribution and high dispersity with the resulting oxidized conjugated
polymers protecting the gold nanoparticles as stable colloidal solution.
The size of the nanoparticles was controlled by simple variation in the
polyamic acid:AuCl3 molar ratio. Unlike poly(amidoamine) dendrimer-encapsulated
gold nanoparticles that are only stable for 1 day before sedimentation,
the current synthesis and stabilization with polyamic acid allows stability
for several months with no change in dispersion, nanoparticle density,
size distribution or absorption spectra when stored at –20º
C. Furthermore, the polyamic acid-capped gold nanoparticles are formed
in less than 1 hour in a rapid, one-step, single-phase synthesis compared
to those produced in 12 hours to 1 day.11 Also, some current
approaches are performed in two phases and thus require extraction into
the organic medium.The PAA reaction enables the direct formation and stabilization
of the nanoparticles in the organic medium.
Experimental Section
In a typical reaction, AuCl3 crystals (1.5 mg, 5 x 10-6
moles) were dissolved in 6 mLs of dimethylformamide (DMF) solutions containing
various amounts of polyamic acid (0.5 mg, 1.0 mg and 3.0 mg) were left
to stand at room temperature. The resulting solutions contained polyamic
acid:AuCl3 in 1:100, 2:100 and 6:100 mole ratios respectively.
Polyamic acid was synthesized by an adaptation of the method previously
reported by Echigo. Its average molecular weight was estimated to be about
10,000 using gel permeation chromatography.
Synthesis of PAA: Briefly 0.010 mol of 4,4 oxydianiline
(ODA) was dissolved in 125 mL of acetonitrile. 50 mL of acetonitrile containing
0.01 mol of 1,2,4,5-Benzenetetracarboxylic dianhydride (pyromellitic dianhydride
– PMDA) was then dropwise for 1 hr to the stirred ODA solution.
Yellow PAA precipitates were formed and the stirring was continued overnight.
The PAA was filtered and dried at room temperature for 24 hr. The Mw
of PAA was estimated using gel permeation chromatography (GPC) was estimated
to be 10000. All solvation processes were achieved by sonication.
Results & Discussion
The reaction medium gradually changed color from yellow, through orange,
and finally to purple. (Figure 1). A control reaction with no polyamic
acid was run concurrently. There was no color change with this solution
as the
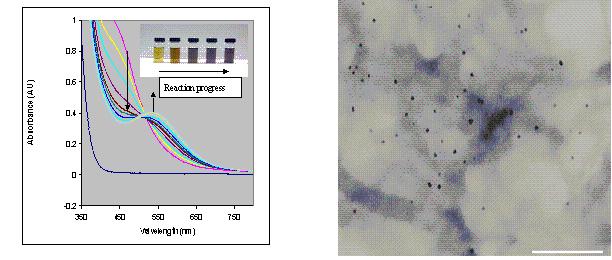
Figure 1: (a) The UV/Vis absorption spectrum for the formation of polyamic acid capped gold nanoparticles; (b) TEM image of reaction resulting from 2:100 mole ratio of polyamic acid:AuCl3 in DMF.
solution retained its original yellow color. The color change in the reaction with the polyamic acid was attributed to the reduction of the AuCl3 by polyamic acid to produce the gold nanoparticles. Polyamic acid is simultaneously oxidized in the process. Figure 1a also shows the uv-visible absorption spectrum of the reaction progress during the formation of the polyamic acid-capped gold nanoparticles in dimethylformamide. It is evident that there is a gradual increase in intensity at 540 nm, indicating the formation of the gold nanoparticles. The shape and position of the final band is characteristic of gold nanoparticles with diameter below 10 nm. This has been attributed to localized surface plasmon oscillation. The isosbestic point at 510 nm is indicative of the involvement of two major species in the reaction medium. The reaction occurred in a similar manner in dimethylsulfoxide and dimethylacetamide although the rate of reaction was relatively much slower in the former solvent.
Figure 1b shows the transmission electron microscopy (TEM) of the PAA-capped gold nanoparticles when a 2:100 mole ratio of polyamic acid:AuCl3 was used for synthesis. The image clearly shows that the nanoparticles are uniformly distributed and well dispersed throughout the polymer. No particle aggregation was seen. This indicated that the p-conjugated polyamic acid stabilized the gold nanoparticle and prevented the aggregation by both steric and electrostatic stabilization mechanisms. The use of conjugation in the stabilizing nanoparticles is a well-known concept. Additional stabilization is also believed to arise from the interaction of the gold with the basic nitrogen centers in the polymer backbone. The average particle size in this case was 5.1 ± 0.9 nm. The nanoparticles were very consistent in size and dispersion pattern. The TEM image and size distribution histogram of the gold nanoparticles when the polyamic acid:AuCl3 mole ratio is increased to 6:100. It is evident that the particles became smaller in size to approximately 4.0 ± 0.7 nm when the polyamic acid:AuCl3 mole ratio was increased to 6:100. This is due to the presence of more polyamic acid molecules that are available for the capping and stabilization of the gold nanoparticles. On the other hand, the reduction of the polyamic acid:AuCl3 mole ratio to 1:100 resulted in slightly larger particles with more aggregation due to less polyamic acid molecules in the reaction medium that is available to stabilize the nanoparticles. The size of the nanoparticles in this case was in the range of 7.8 ± 1.0 nm. This phenomenon of a higher polymer concentration, giving rise to smaller particles has already been observed for polymeric thiols.
This reaction was confirmed using 1HNMR spectroscopy. The
chemical shifts of the hydrogens Ha and Hb in the
reactant polyamic acid and the oxidized polyamic acid were unchanged at
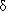 10.5 (see supporting information). The same applied to Hc (
10.5 (see supporting information). The same applied to Hc (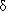 8.3). This meant that part of the polyamic acid molecule remained unchanged
after the reaction. On the other hand the chemical shifts of H
8.3). This meant that part of the polyamic acid molecule remained unchanged
after the reaction. On the other hand the chemical shifts of H (
(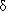 7.7) and He (
7.7) and He (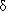 6.8) in the reactant polyamic acid are typical of a para-substituted
benzene aromatic system. However, the nature of the peaks and their chemical
shifts after the reaction changed significantly (
6.8) in the reactant polyamic acid are typical of a para-substituted
benzene aromatic system. However, the nature of the peaks and their chemical
shifts after the reaction changed significantly (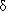 7-
7- 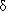 8), and these could be attributed to the
8), and these could be attributed to the  -conjugated
polyamic acid stabilizing the nanoparticles.
-conjugated
polyamic acid stabilizing the nanoparticles.
Metal Sensing and Recovery
The polymer nanoparticle hybrid was initially tested for the determination
of copper using cysteine having strong affinity for the metal of interests.
The effect of polymer- modified gold nanoparticles oxidation time on the
recovery of copper was investigated by keeping all factors constant and
varying the times taken for the polymer oxidation. The recovery of copper
at 10 sec, 100 sec, 200 sec and 500 sec was 34.7%, 75.0%, 83.9% and 99.7%
respectively. These results can be explained by the fact that more polymer
oxidation time increases the number of carbon atoms oxidized to –COOH
moieties thus enabling more cysteine molecules to be covalently bonded.
Based on additional spectroscopic characterization, we believe that there
is significant specificity of the materials towards the metal even when
it is sequestered within the polymers. Further work is required to prove
the feasibility of this technique for other metals.
Summary & Conclusions
In conclusion, we report here the first example in which gold nanoparticles
were prepared using the reduction of AuCl3 by polyamic acid
in organic medium. The polyamic acid was oxidized in the process, thus
resulting in the capping of the gold nanoparticles with the  -conjugated
polyamic acid. Depending on the reactant concentrations, the polyamic
acid-capped gold nanoparticles were synthesized either as well-dispersed
or aggregated particles. Particle size ranged from 4.0 ± 0.7 to
7.8 ± 1.0 nm The size of the particles formed and whether aggregation
of the particles occurs or not depends on the polyamic acid:AuCl3
mole ratio. This method can be applied to the preparation of other PAA-capped
metal nanoparticles and bimetallic particles in organic solvents.
-conjugated
polyamic acid. Depending on the reactant concentrations, the polyamic
acid-capped gold nanoparticles were synthesized either as well-dispersed
or aggregated particles. Particle size ranged from 4.0 ± 0.7 to
7.8 ± 1.0 nm The size of the particles formed and whether aggregation
of the particles occurs or not depends on the polyamic acid:AuCl3
mole ratio. This method can be applied to the preparation of other PAA-capped
metal nanoparticles and bimetallic particles in organic solvents.
Acknowledgement. We thank the Environmental Protection Agency STAR Program for financial support of this research (RD-83090601-0). We also grateful to Henry Eichelberger of the Biological Sciences Department at SUNY-Binghamton for TEM analysis.
