Extramural Research
Presentation Abstract
Grantee Research Project Results
Title of Talk:
Developing Functional Fe0-based Nanoparticles for In Situ Degradation
of DNAPL Chlorinated Organic Solvents
Abstract of Talk:
INTRODUCTION
Dense non-aqueous phase liquids (DNAPLs) in the subsurface remain an important and costly environmental liability. DNAPL serves as a continuous long-term source of groundwater contamination. This project integrates several basic science fields to advance a particle-based strategy for in situ DNAPL degradation by providing targeted delivery of reactive particles directly to the DNAPL.
Over the past decade, laboratory and field studies have demonstrated that zerovalent iron and bimetallic colloids (Fe/Pd) can rapidly transform dissolved chlorinated organic solvents into non-toxic compounds(1). This emerging technology also has the potential to address DNAPL contamination, a vexing contamination problem. The objective of this research is to develop and test reactive nanoscale particles for in situ delivery to, and degradation of, chlorinated solvents that are present as DNAPLs in the subsurface. The hypothesis under consideration is that the surfaces of reactive Fe0-based nanoparticles can be modified with amphiphilic block copolymers to maintain a stable suspension of the particles in water for transport in a porous matrix, as well as create an affinity for the water-DNAPL interface. Delivering reactive particles directly to the surface of the DNAPL-water interface will decompose the pollutant into benign materials, reduce the migration of pollutant during treatment, and reduce the time needed to remove residual pollution by other means, such as natural attenuation.
Research in the first year of the project has focused on i) identifying suitable Fe0 nanoparticles and understanding the properties that control their reactivity with TCE, ii) synthesizing amphiphilic polymer blocks and attaching these blocks to SiO2 nanoparticles, and iii) evaluating the properties of the resulting polymer-modified functional nanoparticles including their hydrodynamic radius, stability, TCE-water partitioning behavior, and mobility in a porous matrix.
RESULTS AND DISCUSSION
Identifying suitable Fe0 nanoparticles and understanding
the properties that control their reactivity with TCE. The TCE reaction
rates, pathways, and efficiency of two types of nanoscale Fe0
particles were measured in batch reactors; particles synthesized from
sodium borohydride reduction of ferrous iron (Fe/B), and commercially
available particles (RNIP) synthesized from the gas phase reduction of
Fe-oxides in H2. Particle characterization indicated many similarities
between the particles, but several distinct differences between the particle
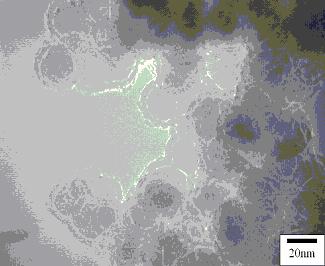
types were found. TEM micrographs of the particles evaluated are given
in Figure 1(a,b). Both particle types showed a core-shell morphology.
RNIP particles had an Fe0 core and a magnetite (Fe3O4)
shell. No other Fe-oxides were detected. Electron diffraction indicated
that the core of the Fe/B (borohydride reduced particles) contains  -Fe0,
however, early reports on metallic nanoparticles made from borohydride
reduction of dissolved iron identified these particles a Fe-B alloy rather
than pure
-Fe0,
however, early reports on metallic nanoparticles made from borohydride
reduction of dissolved iron identified these particles a Fe-B alloy rather
than pure  -Fe0(2,3).
The boron content measured in these particles was ~4 wt% which is nearly
18 mol %. Boron precipitated on the outer shell of the particles (as borate)
accounts for ~0.2-1 wt%, so the remaining boron is most likely present
as a Fe-B alloy as previously suggested, or as distinct phases of Fe0
and B0. No Fe-oxides could be detected on Fe/B, also suggesting
that the shell may be a boron-oxide (borate) rather than a Fe-oxide. The
effect of boron on the reactivity of these particles remains unclear.
-Fe0(2,3).
The boron content measured in these particles was ~4 wt% which is nearly
18 mol %. Boron precipitated on the outer shell of the particles (as borate)
accounts for ~0.2-1 wt%, so the remaining boron is most likely present
as a Fe-B alloy as previously suggested, or as distinct phases of Fe0
and B0. No Fe-oxides could be detected on Fe/B, also suggesting
that the shell may be a boron-oxide (borate) rather than a Fe-oxide. The
effect of boron on the reactivity of these particles remains unclear.
 |
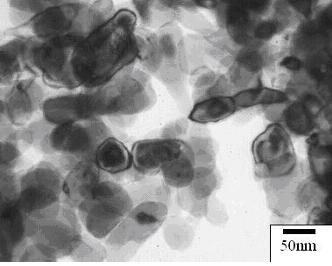 |
Figure 1. |
b) H2 reduction method |
Reactivity was determined under iron limited (high [TCE]) and excess iron (low [TCE]) conditions, and with and without added H2. The reactivity and efficiency of the two particle types were very different and strongly influenced by the oxide shell properties and the presence of boron(4). For example, the main reaction products using Fe/B were primarily saturated (e.g. ethane, butane), while the reaction products using RNIP were primarily unsaturated (e.g. acetylene, ethane). A concentration dependence on the TCE reaction rate and product distribution was observed. Few chlorinated intermediated were observed for either particle. The addition of H2 to the reactor headspace increased the reactivity of Fe/B, and these particles were able to use externally supplied H2 to reduce TCE, suggesting that these particles are catalytic. RNIP particles did not display this behavior. The ability of Fe/B to catalyze the hydrodehalogenation of TCE may be because they are a Fe-B alloy rather than pure Fe0.
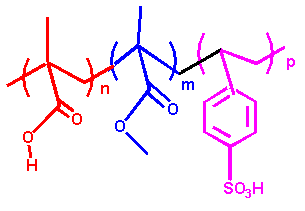
Synthesizing amphiphilic polymer blocks, grafting polymers to SiO2 nanoparticles, and evaluating the properties of the resulting polymer-modified functional nanoparticles. Atom Transfer Radical Polymerization (ATRP) was used to synthesize tailored block copolymers for hybrid nanoparticles(5,6). Concerning the synthesis of Fe0 nanomaterials, we developed a technique for building hydrophobic-hydrophilic hybrids which consist of a short anchoring poly(methacrylic acid) block, a hydrophobic PMMA protective shell, and a hydrophilic SPSt outer block. (Figure 2). Polystyrene and poly(methyl methacrylate) have been identified as good candidates for the hydrophobic blocks. Sulfonated polystyrene has excellent water solubility and makes a good hydrophilic block. Additional methods for grafting polymers to Fe0 are under investigation. ATRP was also used to synthesize particles (dp~100 nm) with an inorganic core (SiO2) and an amphiphilic polymer shell have been synthesized (Figure 3). The particles were water soluble, formed stable suspensions, and partition to the TCE (DNAPL)-water interface. Bench scale transport studies demonstrate that the nanoparticles are readily transported through a saturated porous matrix suggesting that they will be transportable in the subsurface.
 |
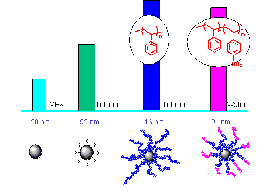 |
| Figure 2. Hydrophobic-hydrophilic | Figure 3. Polymer decorated SiO2
nanoparticles. triblock copolymers containing a short anchoring group |
ACKNOWLEDGEMENTS
The authors thank Dr. Christopher Kim for performing the atomic resolution
microscopy on the Fe0 nanoparticles and the National Center
for Electron Microscopy for the microscope time. The authors also thank
the U.S. Environmental Protection Agency (R830898) and the Department
of Energy (DE-FG07-02ER63507) for their financial support.
REFERENCES
1. Zhang, Wei-xian, J. Nanoparticle Research, 2003, 5(3-4), 323-332.
2. Wonterghem, J., Morup, S., Koch, C., Charles, S., Wells, S., Nature,
1986, 322, 622-623.
3. Shen, J., Li, Z., Yan. Q., Chen. Y., J. Phys. Chem., 1993, 97, 8504-8511.
4. Liu Y., Majetich, S. A., Tilton, R. D., Sholl, D. S., Lowry, G.V.,
¡§TCE Dechlorination Rates, Pathways, and Efficiency of Nanoscale
Iron Particles with Different Properties¡¨, submitted to Environ.
Sci. &Technol. May 31, 2004.
5. Wang, J. S.; Matyjaszewski, K. J. Am. Chem. Soc. 1995, 117, 5614.
6. Matyjaszewski, K.; Wang, J. S., US 5,763,548, 1995.
