Extramural Research
Characterizing Risk at Metal Finishing Facilities
Grantee Research Project Results
27 January 1998
Dennis J. Brown, Ph.D.
Parsons Engineering Science, Inc.
2101 Webster Street, Suite 700
Oakland, CA 94612
A draft of this report was prepared during the author’s tenure as a Fellow under the American Association for the Advancement of Science/U.S. Environmental Protection Agency Fellowship Program during the summer of 1997. Since that time significant changes have been made to the report in response to comments received from technical reviewers both from within and from outside of EPA. The views expressed herein are entirely the author’s and do not represent official policy of either the EPA or AAAS. The report is subject to further review and revision. Mention of trade names of commercial products does not constitute endorsement or recommendation.
ACKNOWLEDGMENTS
The author wishes to thank the AAAS and the EPA for providing the opportunity to pursue the work described herein and for supporting the 10-week Summer Environmental Science and Engineering Fellowship Program. The author also wishes to thank his mentor, Paul Shapiro, for his guidance, helpful discussions, and review of the work both during and after the fellowship period. The author is grateful to the following persons who provided assistance during the fellowship: Babasaheb Sonawane and the staff of EPA’s National Center for Environmental Assessment for providing technical support and the resources to conduct this work; Michael Callahan, John Schaum, and Herman Gibb for reviewing and commenting on various versions of this document; Peter Gallerani, for discussing his work on metal finishing facility emissions and for arranging a tour of the B.F. Goodrich metal finishing operation in Vergennes, Vermont; Ronald Vezzi, B.F. Goodrich Process Engineer; Karen Morehouse, EPA Fellowship Coordinator; and Claudia Sturges, AAAS Fellowship Program Manager. The author is grateful to the National Metal Finishing Resource Center for providing information on the metal finishing industry.
Among those who provided useful comments on the earlier draft report the author wishes to thank Caroline Freeman and William Perry, Occupational Safety and Health Administration; Amy Vasu and Ted Palma, EPA, Office of Air Quality Planning and Standards; Peter Preuss and Richard Walentowicz, EPA, ORD, National Center for Environmental Research and Quality Assurance; Dean Menke, 1997/1998 AAAS/EPA Risk Assessment Fellow; Chris Branson, National Institute of Standards and Technology/Industrial Technology Institute Manufacturing Extension Center; and Joel Barnhart, American Chrome and Chemicals Company and the Chrome Coalition.
TABLE OF CONTENTS
1.1 New Approaches To Environmental Protection
2. Risk Assessment For Metal Finishing Facilities
2.1 The Risk Assessment Process
2.2 Assessing Risk For Chromium Electroplating Facilities
APPENDIX A Output from Risk Assistantä A-1
ABSTRACT
Facility-based risk characterization for workers and surrounding communities is a high priority issue for stakeholders in the Environmental Protection Agency’s Common Sense Initiative Metal Finishing Sector. Platers, environmental groups, community groups, labor, and regulators all need and want to know what emissions are coming out and in what amounts from metal finishing operations. They also want to know what health risks those emissions create for workers and the surrounding communities.
A process is described herein that includes a problem formulation phase to identify the types and forms of information that are wanted by the different stakeholders and a risk assessment phase to quantify the health risks associated with facility emissions. A screening level risk assessment is performed in which toxicity information and exposure data are used to show how a facility-based risk assessment could be performed for a typical electroplating operation. Information needs for a more refined assessment are presented.
A single iteration of the problem formulation and risk assessment processes may lead directly to a risk management decision or the steps may be modified and repeated, taking into account input from stakeholders obtained during the risk communication process. Uncertainties associated with toxicity information and exposure scenarios will present challenges for providing simple (but not simplistic) methods of risk assessment that can be applied by facility operators, community groups, and other stakeholders. This type of risk characterization is not only desired but possible to carry out for a variety of exposure scenarios.
1. Introduction
The mission of the United States Environmental Protection Agency (EPA) is to protect and improve the quality of public health and the environment. As applied to industrial sources of pollution, this mission has been carried out primarily through the development and implementation of policies and programs to reduce or prevent releases of chemicals to the environment. Many of these policies and programs focus on media-specific (i.e., air, water, soil) controls on emissions through the authority granted the EPA by various regulatory statutes (e.g., the Clean Air Act, the Clean Water Act, and the Resource Conservation and Recovery Act). The EPA and others have recently suggested that the current regulatory system, which uses a chemical-by-chemical, medium-by-medium, risk-by-risk approach to assess and reduce environmental health risks, be modified to consider health and environmental effects of pollutants in their broader context, which often includes emissions to more than one medium and/or exposures of multiple populations (e.g., on-site workers, the public) (Browner, 1994; Presidential/Congressional Commission on Risk Assessment and Risk Management, 1997a, 1997b).
1.1 New Approaches To Environmental Protection
To aid in the development and assessment of new approaches to environmental protection, EPA has sought input from persons who may be affected by any change in EPA’s regulatory process through programs such as the Common Sense Initiative (CSI) and Project XL. These persons (stakeholders) may include representatives from industry, workers, trade organizations, community groups, environmental justice groups, environmental groups, and state and local governments. It is EPA’s stated purpose to use the input from these groups to reinvent the way that it accomplishes environmental protection (Browner, 1994). One form that reinvention has taken is to move away from "command-and-control" policies toward the greater use of performance-based approaches that reward environmental excellence as much as they punish non-compliance (EPA, 1997b). The EPA has developed several pollution prevention programs that encourage industry to reduce the quantity of hazardous chemicals released to the environment by substituting less toxic chemicals in their operations, by using smaller quantities of chemicals, or by recycling or reusing chemicals within their manufacturing operations (EPA, 1995d).
As measured by reduced total emissions of toxic chemicals to the air, soil, and water, pollution prevention programs have been successful (EPA, 1997c). However, a reduction in total emissions reveals only part of the story. Reduced emissions do not necessarily equate to a proportional reduction of health risks. The relative change in health risks may be disproportionately larger or smaller than the reduction in total emissions because health risks are a function of both the degree of exposure to toxic chemicals and the nature and intensity of the chemicals’ toxic effects. With regards to exposure, reducing emissions where there is little or no exposure would yield a smaller health benefit relative to reducing emissions where there is much potential exposure. With regards to toxicity, a large decrease in the emission of a chemical of relatively low toxicity may have a smaller health benefit than a small decrease in a more toxic compound. Only by describing emissions in terms of both exposure and toxicity can any health benefits from reduced emissions be determined.
Risk assessment is the process of estimating the chance that adverse health effects will result from exposure to a chemical, biological, or physical agent. Human health risk assessment attempts to quantify the adverse health effects by identifying the nature of the potential injury (hazard identification) and the populations that are at risk (exposure assessment), by measuring the relationship between a given exposure and the potential injury (dose-response assessment), and by combining these three pieces of information to estimate the probability that harm will occur (risk characterization) (NRC, 1983; NRC 1994). Risk assessment is used by EPA and others as one input to making risk management decisions.
Risk management is the process of identifying, evaluating, selecting, and implementing actions to reduce risk to human health and to ecosystems. The goal of risk management is to identify and implement actions that reduce or prevent risks while taking into account social, cultural, ethical, political, and legal considerations. A recent report by the Presidential/Congressional Commission on Risk Assessment and Risk Management (1997a, 1997b) emphasizes the importance of engaging stakeholders throughout the risk assessment/risk management process to assure that risk managers--e.g., government officials--take into account these considerations to achieve good risk management decisions.
The development of a better understanding of the risks to workers and surrounding communities associated with emissions from individual facilities is a high priority issue for stakeholders in EPA’s CSI Metal Finishing Sector (EPA, 1997a). Platers, environmental groups, community groups, labor, and regulators all need and want to know what emissions are being produced and in what amounts by metal finishing operations. They also want to know what health risks those emissions create for workers and the surrounding communities. The process of risk assessment can provide a tool for stakeholders associated with the metal finishing industry to better understand and evaluate the human health effects associated with chemicals emitted by metal finishing facilities. The next two sections provide a brief overview of the metal finishing industry and discuss the forum (i.e., the EPA’s Common Sense Initiative) that provided the impetus for this project.
1.2 The Metal Finishing Industry
The metal finishing industry encompasses a broad range of processes that are performed on manufactured parts, usually after they have been shaped and machined (Murphy, 1996; EPA, 1995a, 1995c). These processes generally alter the surface of the article to lend it properties not possessed in its "unfinished state." The processes most commonly impart a decorative finish on the article or provide it with additional functional characteristics such as corrosion resistance. Common metal finishing operations include electroplating, electroless plating, anodizing, conversion coating, and painting (Murphy, 1996). Additional steps that may be performed before or after finishing operations include cleaning (e.g., degreasing with organic solvents), etching, and corrosion protection. Many of these processes (e.g., electroplating, electroless plating, and anodizing) involve the immersion of the metal parts through a series of liquid baths containing solutions that impart the desired finish.
The metal finishing industry is comprised of both "job shops," mostly small businesses with limited capital and personnel, and "captive" metal finishing operations within larger manufacturing facilities. Job shops perform finishing processes on parts that they receive from outside sources, whereas captive shops perform finishing processes on parts that their firms manufacture. Captive shops are typically involved in the manufacture of such items as machinery, automobiles, appliances, and musical instruments. Job shops involved in metal finishing are classified primarily under the Department of Commerce’s Standard Industrial Classification (SIC) Code 3471, metal plating and polishing. Manufacturing facilities that incorporate captive shops are generally classified within SIC Codes 34 through 39, which encompass facilities that fabricate metal products.
Estimates of the number of metal finishing facilities varies somewhat depending upon how the industry is defined, but it is believed to include about 3,000 job shops and about 10,000 captive shops (EPA, 1997d). The typical job shop is about 30 years old, employs about 10 to 20 people and has annual net sales of approximately $1.1 million (NCMS, 1994; CAMP, 1995). Facilities can be found throughout the country, but are concentrated in industrialized areas in the Northeast, Midwest, Texas, and California.
Metal finishing facilities release a variety of toxic compounds (EPA, 1995a). Chlorinated hydrocarbons are emitted during cleaning (degreasing) of metal parts; caustic mists, cyanides, and metals are released from electroplating operations; and volatile organic compounds are emitted during painting. The emitted chemicals can cause a variety of adverse health effects depending upon the toxic nature of the chemical; the medium of exposure (i.e., air, water, soil, or food); the chemical concentration to which an individual is exposed; and the duration and frequency of the exposure. In addition, exposed individuals will have varying degrees of sensitivity to chemicals depending upon the person’s health status, age, and sex. Adverse health effects may include cancer (hexavalent chromium, benzene), developmental toxicity (lead, mercury, glycols), neurotoxicity (solvents, mercury), chemical burns (acids and alkalis), or dermal, respiratory, or eye irritation (acid vapors, solvents, metals) (Klaasen, 1995).
Table 1-1 lists some of the chemicals that may be found in metal finishing process emissions (EPA, 1995a). The list is not a description of the actual emissions from any specific facility. Emissions from each facility are dependent upon the processes performed and the types and effectiveness of any pollution control practices and control devices that are used at the facility. By combining emissions data with chemical exposure and toxicity information in the risk assessment process, an evaluation of the human health effects of emissions can be made.
1.3 Common Sense Initiative
CSI is an attempt by EPA to take a new approach to creating policies and environmental management solutions for American industries (Browner, 1994). Participants in the CSI program have been asked to work together to achieve environmental protection on an industry-by-industry basis. Stakeholders associated with six industrial sectors--automobile manufacturing, computers and electronics, iron and steel, printing, metal finishing, and petroleum refining--currently participate in the CSI program. These six sectors represent a cross-section of American industry and taken together they comprise over 11% of the U.S. Gross National Product; employ over 4 million people; and account for about 12% of reported releases of toxic substances. Because of the importance of these industries as measured by these characteristics and because of their diversity in size, products, and operations, EPA believes that they offer an excellent opportunity to create environmental solutions that can operate across industries and to expand CSI to other sectors (EPA, 1994).
Table 1-1
Chemical Substances Potentially Used in, Generated by, or Emitted
from Metal Finishing Facilities
|
Metals and Metal Compounds Aluminum Arsenic, arsenic disulfide Barium Cadmium, cadmium acetate, cadmium chloride Chromium, chromic acid Copper Iron Lead Manganese Mercury Nickel, nickel acetate, nickel sulfate Nickel-cobalt acetate Selenium Silver Tin-lead Zinc Alkalis Sodium hydroxide Cyanides Potassium cyanide Sodium cyanide Zinc cyanide Strong Acids Hydrochloric acid Hydrofluoric acid Nitric acid Phosphoric acid Sulfuric acid Weak Acids Acetic acid Citric acid Oxalic acid Tartaric acid Other Inorganics Chlorine Fluoride Potassium nitrate Sulfur dioxide |
Organics Acetone Acetone cyanohydrin Benzene Carbon disulfide Carbon tetrachloride Chlorinated fluorocarbons, 1,1,2-trichloro-1,2,2-trifluoroethane (Freon-113), Trichlorofluoro-methane Chlorobenzene Chloroform Cresols (cresylic acid) Dichloromethane (methylene chloride) Ethyl acetate Ethyl benzene Ethyl ether 2-ethoxyethanol Formaldehyde Glycols Isobutanol Kerosene Ketones, cyclohexanone, methyl ethyl ketone, methyl isobutyl ketone Methanol Mineral oil Naphtha n-butyl alcohol Nitrobenzene 2-nitropropane 1,2-dichlorobenzene Phenol Pyridine Tetrachloroethylene Toluene 1,2,4-trichlorobenzene 1,1,1-trichloroethane 1,1,2-trichloroethane Trichloroethylene Xylene |
(Modified from USEPA, 1995c)
The overall direction of CSI is determined by stakeholders from each industry sector. A CSI Council, comprised of high-level decision-makers from all stakeholder groups and across all involved industrial sectors, provides a forum for the exchange of ideas among sectors (Figure 1-1). The CSI Council is chaired by the EPA Administrator. For each industrial sector in CSI, EPA also has convened a team of stakeholders (i.e., a sector-specific subcommittee) that looks for opportunities to create new sector-specific alternatives to the current regulatory system to achieve greater environmental gains at less cost to industries and taxpayers (a process sometimes labeled "cleaner, cheaper, smarter") (EPA, 1994).
Figure 1-1
Metal Finishing Sector OrganizationWithin the Common Sense Initiative
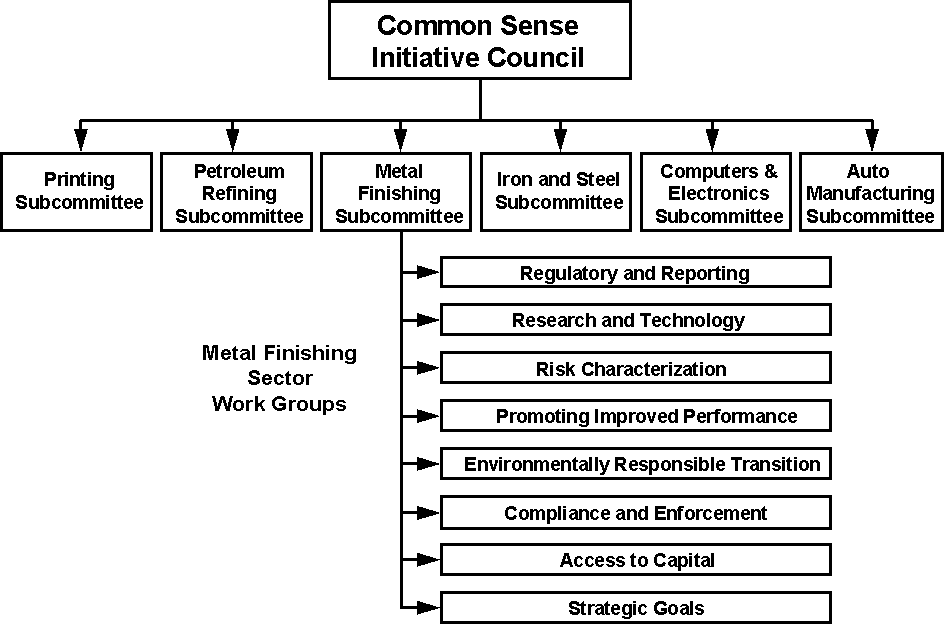
EPA hopes that CSI, with its involvement of a diverse and comprehensive group of stakeholders, will serve as a model for changing the environmental protection process from one of conflict to one of collaboration and consensus. Historically, much of EPA’s rule-making has resulted in litigation. This process diverts valuable resources of all parties from the work of protecting the environment and public health. The EPA hopes CSI will provide a forum within which former adversaries will become partners in protecting the environment. To assure communication among stakeholders, sector subcommittees have met regularly since CSI’s inception to identify and refine their objectives, to plan projects to meet those objectives, and to discuss progress in the various projects underway, policy considerations, and other issues (EPA, 1997d).
Each industry sector subcommittee has been asked to explore common issues, including alternative regulatory systems, pollution prevention, reporting, compliance, permitting, and environmental technology, that may have broad applicability to their sector as well as to other industry sectors. EPA hopes that the new systems will be more flexible, will encourage innovation, and will be tailored to the needs of the industry and its environmental problems, while at the same time the systems will encourage public participation, provide information about facilities’ environmental performance, and meet or exceed legal requirements (EPA, 1994).
The Metal Finishing Subcommittee has about 24 members representing metal finishing companies, trade associations, suppliers, environmental and community groups, organized labor and state and local governments. Representative organizations include the American Electroplaters and Surface Finishers Society (AESF), the Natural Resources Defense Council (NRDC), the United Auto Workers (UAW), the Barrio Planners of Los Angeles, the Water Environment Federation, and the Association of Municipal Sewerage Agencies (AMSA). Members of the subcommittee have identified a set of National Performance Goals for the sector that include three facility-based performance goals:
- Reduction in hazardous emissions and exposures ("cleaner");
- Increased economic payback and decreased costs ("cheaper");
- Improved resource utilization ("smarter");
and two sector-wide performance goals:
- Industry-wide achievement of the facility-based goals;
- Industry-wide compliance with environmental performance requirements (EPA, 1997b).
To meet these goals, the subcommittee has endorsed 14 projects, and supports an additional CSI small business sector project. It has created eight work groups (Figure 1-1) to carry out these projects and to identify important needs for the sector.
The Metal Finishing Subcommittee’s Research and Technology Work Group examines and provides information about new technologies for the metal finishing industry and seeks to better understand the technology needs of the metal finishing industry, as a basis for tailoring Federal and private sector research and development to meet those needs. The work group’s desired end product is a "customer-oriented" research and development strategy for the industry. The work group’s objectives are to assure that research efforts (including technology transfer and diffusion) will address the most significant environmental needs of the metal finishing industry; that the results will be accessible to the typical metal finishing job shop; and that the research program will focus on pollution prevention and remediation technologies, so as to be of greatest benefit to small job shops, some of which are located in brownfield areas.
The work described herein was undertaken in response to one of eight priority research needs identified by the Research and Technology Work Group in its National Metal Finishing Environmental R&D Plan (R&D Plan) (EPA, 1997a). The eight priority research needs were identified from among 74 projects that were rated by 27 experts from all metal finishing sector stakeholder groups. The experts rated the research needs according to three criteria--the likely impact of the project in achieving or exceeding Federal, state, and local compliance requirements; achieving widespread adoption within the industry; and reducing risks to workers, the surrounding community, and the environment. The rating system was used to prioritize research and development needs and to make recommendations for the highest priority research areas.
The eight specific recommendations for further research that were made in the R&D Plan include:
- Develop and apply simple methods to describe the emissions from plating operations and use these values to characterize risks to workers, surrounding communities, and the environment;
- Continue and expand research and development on various aspects of reducing and eliminating multi-media emissions from hexavalent chromium plating operations;
- Focus research and development on reducing cyanide emissions and on developing improved analytic methods to determine the presence, concentration, and impacts of cyanide in waste streams;
- Demonstrate methods of off-site recovery of metals, acids, and cleaners;
- Focus research and development on low emission and emissionless chlorinated solvent vapor degreasing systems for metal plating operations and on evaluating alternatives to chlorinated solvents for cleaning--especially new, alternative cleaners that have recently come on the market;
- Develop a rapid verification protocol that provides information on technology performance, cost and maintenance requirements on which companies could base decisions to purchase technologies;
- Conduct research and development to reduce cadmium emissions and to seek alternatives to its use; and
- Develop and disseminate short, well-researched, peer-reviewed articles on the selection and use of simple technologies for improved environmental performance.
Among the goals in the R&D Plan the highest priority was given to developing and applying simple methods to characterize the emissions from plating operations (and more specifically, chromium electroplating operations) and to use the output from these methods to characterize the health risks to workers, surrounding communities, and the environment (EPA, 1997a). To help attain this goal the objectives of this project are:
- to identify the types and sources of information that are needed to assess risks to workers and surrounding communities from metal finishing facilities;
- to develop a general facility model that describes potential human exposure pathways;
- to present equations that characterize the exposure pathways from emission sources to workers and the public; and
- to quantify the lifetime excess cancer risk and potential for other health hazards from hexavalent chromium in a screening risk assessment process.
By explaining the steps performed and the data needed to conduct a risk assessment we hope to assist those associated with the industry to better understand the risk assessment process and the questions that can be answered by the process.
Although this report focuses on the potential effects that emissions from metal finishing operations may have on human health, emissions from some facilities may also affect ecological receptors. An ecological risk assessment can be performed for those facilities where ecological receptors may be at risk beginning with a problem formulation phase that develops a site conceptual model for the ecological receptors at risk and identifies the questions that the risk assessment process would be designed to answer (EPA, 1996b).
2 RISK ASSESSMENT FOR METAL FINISHING FACILITIES
2.1 THE RISK ASSESSMENT PROCESS
Human health risk assessment is one part of the process that begins with the recognition that a potential health problem exists (Problem Formulation) and continues to a decision by risk managers to take actions (Risk Management) to reduce or eliminate any identified potential for harm (Figure 2-1). Risk assessment entails the evaluation of information on the hazardous properties of chemicals and the extent of human exposure and the characterization of the resulting risks. The complete risk assessment process is comprised of four steps--hazard identification, dose-response assessment, exposure assessment, and risk characterization (NRC, 1983; 1994).
Figure 2-1
The Risk Assessment/Risk Management Paradigm
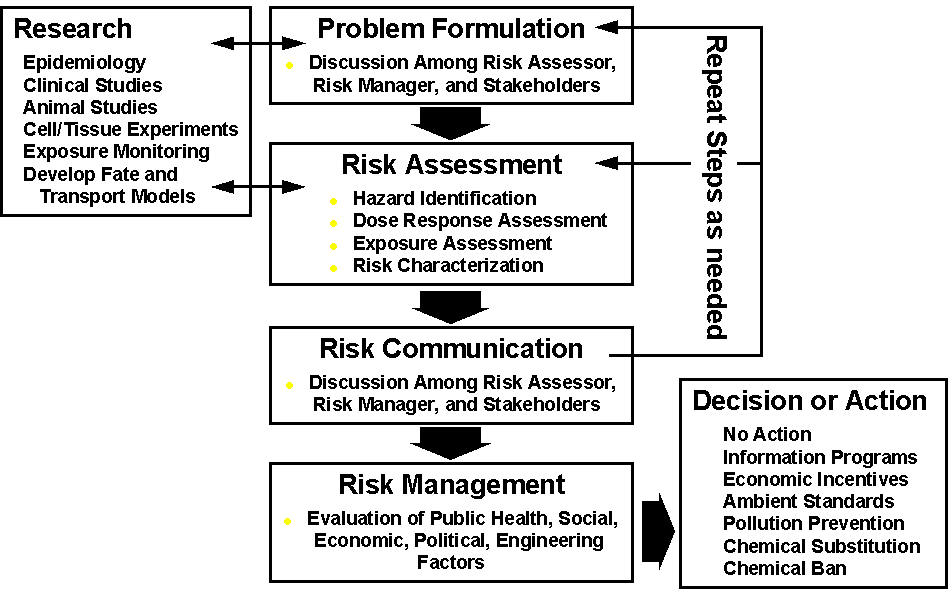
(Based on NRC, 1983; 1994)
Although complete risk assessments contain all four steps, they may nevertheless involve varying levels of effort. Risk assessments are often performed using a phased approach in which upper estimates of exposure and chemical concentrations, which are believed to be conservative (i.e., health protective) and likely to overestimate risk, are first used to assess risk. A phased approach to risk assessment allows the risk assessor to identify those health risks that are potentially the most important and consequently to eliminate from further evaluation those chemical exposures that clearly do not present a health risk. This approach simplifies and focuses subsequent phases of analysis and at the same time reduces the cost and time to perform the risk assessment, especially at facilities with relatively low levels of emissions or emissions of relatively non-toxic compounds.
Problem formulation is a useful process to perform prior to the risk assessment process because it provides an opportunity to gather input from all stakeholders prior to commencing any analysis. The purpose of the problem formulation process is to sharpen the focus of the risk assessment on those problems of greatest concern to those persons, risk managers and stakeholders alike, who will be using the information. Problem formulation may involve a statement of the suspected problem based on available information; the identification of questions to be answered by the risk assessment; the identification of any research that may be needed before beginning the risk assessment; and/or the presentation, review, and comment on an analysis plan for the risk assessment process. Once the objectives of the risk assessment process have been identified the four steps of risk assessment can be conducted.
The first step of the risk assessment process, hazard identification, seeks to identify the potential health effects (e.g., dermal irritation, neurotoxicity, cancer, reproductive toxicant) that may result from exposure to a chemical or physical agent. This information is gathered from the health effects literature, which may provide evidence either for or against the agent as the cause of a specific type of health effect. Such studies often characterize the behavior of a chemical within the body and its interactions with organs, cells, or even parts of cells. Data regarding these interactions may be of value in answering the ultimate question of whether the forms of toxicity observed in an epidemiology study, population group, or test animal are also likely to occur as a result of an environmental exposure.
For any harmful effect that is identified, the second step of the risk assessment process, dose-response assessment, is conducted. Dose-response assessment attempts to determine the relationship between the quantity of substance to which an individual is exposed and the severity of the adverse health effect. Dose-response data are derived from animal studies or, less frequently, from studies in exposed human populations (e.g., epidemiological studies of workers have been performed for chemicals of concern to industry). There may be several dose-response relationships for a substance because it may induce more than one type of harmful effect or it may induce different effects via different pathways of exposure. The level of confidence in an estimate of a dose-response relationship is partly dependent upon the source of data used to derive the estimate. Generally, estimates derived from human studies, such as epidemiological studies of workers, contain less uncertainty than those derived from animal studies. The first two steps of the risk assessment process are chemical (substance) specific. Once completed, the information can be used again in many assessments of various "real-life" situations.
The third step of the risk assessment process, exposure assessment, seeks to characterize "real-life" situations by determining the intensity, frequency, and duration of exposures to the chemical substance(s) in question that are known to occur or could occur in the future. Exposure assessments can evaluate past, present, or future exposures and may involve either direct or indirect assessments of exposure. Direct assessments measure the contact between the exposed person(s) and the substance(s) being studied through the use of personal monitors (e.g., a small air pump and filter that collects air contaminants from within the breathing zone of the person being studied). Indirect assessment uses measurements of concentrations and other data in the physical surroundings of the people being studied (e.g., air flow direction and rate), along with information about where the people are and what they are doing to bring themselves into contact with the substance(s).
Risk characterization, the final step of the risk assessment process, combines the assessments of hazard, dose-response, and exposure to estimate the probability of the occurrence of a specific adverse effect in an exposed individual or population. The results of the risk characterization are then communicated to risk managers and other interested parties with an overall analysis of the quality of the information in the assessment (NRC, 1994). The uncertainty associated with the risk assessment and the sources of this uncertainty are presented. The uncertainty analysis should indicate whether the assumptions made in the preceding steps tend to under- or over-estimate the level of risk.
Risk assessment is closely linked but distinct from risk management, the process by which the results of a risk assessment are integrated with political, social, economic, and engineering considerations to arrive at decisions about the need and methods for reducing risk. A risk assessment should be prepared, therefore, with both risk managers and stakeholders in mind, to assure that appropriate information will be provided in a format that is understandable and useable to all interested parties. Maintaining communication among risk managers, stakeholders, and the risk assessor throughout the risk assessment process will increase the likelihood that all interested parties will remain engaged in the process and will contribute input on political, social, and economic issues that are part of the risk management process.
2.1.1 Hazard IdentificationThe first step of the risk assessment process at a metal finishing facility is to identify the chemicals present that may affect human health (Table 2-1). Chemicals that may be used, stored, or generated at the facility from all potential sources including raw materials, process intermediates, and waste products should be considered. A preliminary list of the hazardous substances that may be associated with metal finishing processes has been compiled (Table 1-1) (EPA, 1995a). The actual list of chemicals at any given facility will consist of a subset of this list, possibly supplemented with additional chemicals (e.g., as technology innovations occur and new chemical processes are developed, additional chemicals may require evaluation). Raw materials could be identified from company purchasing records or from the facility’s Material Safety Data Sheets; process intermediates could be identified by evaluating the chemical processes that are conducted at the facility; and waste products could be identified from government-required reporting forms such as EPA forms for the reporting of chemicals to the Toxic Release Inventory.
Once a facility-specific list of chemicals is compiled, the adverse health effects associated with the chemicals can be identified by reviewing existing toxicological information. One source of this information is EPA’s Integrated Risk Information System (IRIS), which is available on the internet (www.epa.gov/ncea/iris.htm). It is possible at this step of the risk assessment that some chemicals will be identified for which little or no toxicological information is available. Lack of such information is a source of uncertainty in the risk assessment. Additional laboratory or epidemiological research on the chemicals may be needed to reduce this uncertainty.
Table 2-1
Principle Steps of the Risk Assessment Process
for Metal Finishing Facilities
|
Risk Assessment Step |
Associated Tasks |
|
Hazard Identification |
Identify chemicals emitted or released from known chemical usage and emissions data from the facility or industrial sector. Identify unwanted health effects of chemicals that are emitted or released. |
|
Dose-Response Assessment |
Identify cancer potency factors, unit risks, reference concentrations, and reference doses for each chemical as derived by EPA or by others from animal studies and human epidemiological studies. Verify assumptions used in existing data with historical information about the facility or industrial sector. |
|
Exposure Assessment |
Collect plant emissions information from permits, emissions reports, interviews, and industry records. Characterize any variations in emissions over time (e.g., daily or longer term fluctuations that result from variations in facility schedules and production runs). For workers, describe work environment, such as ventilation in rooms, air exchange rates, direction of air flow, and whether any special systems are present to prevent or reduce worker exposure (including any personal protective equipment that workers may be required to wear). Describe design, placement and effectiveness of building ventilation system. For more refined assessments of exposure pathways in air, obtain meteorological data from weather service and air districts (commonly available for use in EPA air models) and predict concentrations of pollutants in air at varying distances from the emission source using air dispersion model(s). For more refined assessments of exposure pathways in water, collect hydrogeological data from United States Geological Survey (USGS), water districts, or state and county governments and use to predict the movement of chemicals in groundwater and surface water. Collect demographic information from census information, county and city records, and site surveillance. Verify concentrations of chemicals in environmental media through workplace and/or community observation and/or monitoring. Describe how emissions controls and environmental fate and transport processes affect exposure concentrations for off-site populations. |
|
Risk Characterization |
Describe the probability of unwanted health effects by combining information from hazard identification, dose-response assessment, and exposure assessment steps. Identify and discuss sources of uncertainty and variability associated with variables in the risk assessment. |
(Based on Schaum, 1997)
2.1.2 Dose-Response AssessmentThe dose-response assessment attempts to determine the relationship between the quantity of substance ingested, inhaled, and/or absorbed (e.g., through the skin) and the probability of the occurrence and severity of an adverse health effect. The dose-response assessment considers whether sensitive or special populations, such as children, persons with compromised immune systems, or the elderly may be more susceptible to a chemical’s harmful effects. Generally, dose-response information is gathered from two types of sources--animal studies and human epidemiological studies. Dose-response values derived from both types of studies contain some degree of uncertainty. Values that are based on animal studies are extrapolated from the responses of test animals that are exposed under laboratory conditions, often at relatively high concentrations. Adjustments must be made to the experimentally-derived dose-response values to obtain a dose-response value appropriate for humans. The adjustments account for metabolic and physiological differences between humans and animals, differences in exposure duration and intensity, and differences in exposure pathways (e.g., ingestion in food versus ingestion in water). Values that are based on studies of human populations contain less uncertainty but still often require extrapolation from a high dose, short-term exposure, such as occurs from accidental exposure or an intentional overdose, to a low dose, chronic exposure. When the studied population used to derive the dose-response value is similar to the population of interest in the risk assessment (e.g., epidemiological studies of workers used to derive safe workplace concentrations) the estimates of dose-response are more certain.
EPA’s IRIS database contains dose-response information for over 500 specific chemical substances. The database was initially developed for EPA staff in response to demand for consistent information on chemical substances for use in risk assessments, decision-making, and regulatory activities. Dose-response values in the database represent EPA consensus scientific positions on potential adverse human health effects that may result from chronic (e.g., lifetime) exposure to environmental contaminants. This information has been evaluated by scientists from EPA’s program offices and Office of Research and Development (ORD) who are experienced in issues related to both the qualitative and quantitative risk assessment of carcinogenic and toxic agents. The review process leads to an internal EPA scientific consensus regarding risk assessment information on a chemical.
Dose-Response Values for Noncarcinogenic Effects. One widely held view among toxicologists is that many of the harmful effects that result from exposure to toxic chemicals occur only when an individual’s exposure (via inhalation, ingestion, and/or dermal contact) exceeds some threshold level of uptake. The threshold level is generally expressed as a chemical dose (e.g., reference dose, or RfD) to which an individual may be exposed over a portion of a lifetime (subchronic RfD) or during a lifetime (chronic RfD) without an appreciable risk of adverse effects. The chemical dose is expressed as the weight of chemical per kilogram of body weight per day (e.g., milligrams/kilogram*day or mg/kg*day). For chemicals in air the threshold level is expressed as a concentration (reference concentration, or RfC) such as micrograms of chemical per cubic meter of air (mg/m3).
The dose-response values noncarcinogenic effects are based on toxicological information derived from either animal or human studies. Chemical exposure in these studies may be acute (a brief exposure of a few minutes to a few days), subchronic (a few weeks to months), or chronic (usually includes at least a tenth of the life span of a species, generally six months or more). Chronic exposures generally have the lowest thresholds for adverse effects and are most commonly used to derive chemical RfDs and RfCs for EPA risk assessments. However, if emissions are episodic or of short duration, the RfDs and RfCs that relate to acute exposures may be appropriate to use in the risk assessment. The RfDs and RfCs cited in this report were derived by EPA’s program offices and ORD and have been reported in the IRIS database or in EPA’s Health Effects Assessment Summary Tables (EPA 1995b; 1997e) (Table 2-2). These values are estimates (with uncertainty spanning perhaps an order of magnitude) of continuous exposure to the human population that are likely to be without appreciable risk of deleterious effects during a lifetime.
EPA’s RfDs and RfCs have been calculated to be protective of sensitive members of human populations. A margin of safety has been applied to derive RfDs and RfCs from experimental data. This margin of safety is applied to account for intra- and inter-species variations, for limited or incomplete data, for evaluating the significance of adverse effects, and for adequately protecting sensitive human populations. In practice, the experimentally-derived values are divided by an uncertainty factor (generally, a number between 3 and 1,000) and possibly by an additional modifying factor to add this margin of safety to the RfDs and RfCs. EPA’s rationale for the application of these safety factors is given in the IRIS database for each chemical where a RfD or RfC is given.
Dose-Response Values for Carcinogenic Effects. EPA considers the weight of evidence that a chemical is a carcinogen and for chemicals that are known or likely to cause cancer, the agency calculates an oral slope factor and/or inhalation unit risk (EPA, 1986; 1996a). Oral slope factors and unit risks are estimates of the relationship between dose or concentration and the probability that a chemical will induce cancer. Oral slope factors are upper bound estimates of the cancer risk per unit intake of a chemical over a person’s lifetime. Inhalation unit risks are upper bound estimates of the cancer risk per unit of concentration of a chemical in air over a person’s lifetime. Because the slope factors and unit risks are upper bound estimates, the risk of cancer for an exposed individual over a lifetime is unlikely to exceed the calculated probability and likely will be less. Oral slope factors and/or inhalation unit risks are given in Table 2-2 for each chemical where information is available. Slope factors are expressed as the inverse of the dose, "(mg/kg*day)-1," while unit risks are expressed as the inverse of concentration, "(mg/m3)-1." Slope factors and unit risks in this report were derived by EPA’s program offices and ORD. Some states, notably California, have derived slope factors that differ from those derived by EPA (DTSC, 1994b).
The dose-response estimates have numerous uncertainties, including those associated with extrapolations from animal data to humans and from high experimental doses to lower environmental exposures. These uncertainties may span an order of magnitude or more. Actual incidence of health effects is influenced by the physiology and health-status of the exposed individual or populations such as general health, age, and sex and by the degree of exposure to the chemical, which is estimated during the exposure assessment step of the risk assessment (EPA, 1993).
Table 2-2
Published EPA Human Health Toxicity Values for Chemical
Substances
Potentially Emitted by Metal Finishing Processes
|
RfC |
unit risk |
RfDo |
SFo |
RfC |
unit risk |
RfDo |
SFo |
||
|
Chemical |
mg/m3 |
(mg/m3)-1 |
mg/kg/day |
(mg/kg/day)-1 |
Chemical |
mg/m3 |
(mg/m3)-1 |
(mg/kg/day) |
(mg/kg/day)-1 |
|
Metals (1) |
Silver |
n.d. |
n.a. |
5.0 x 10-3 |
n.a. |
||||
|
Aluminum |
n.d. (2) |
n.a. (2) |
1.0 x 101 |
n.a. |
Tin |
n.d. |
n.a. |
6.0 x 10-1 |
n.a. |
|
Arsenic |
n.d. |
4.3 x 10-3 |
3.0 x 10-4 |
1.5 x 100 |
Zinc |
n.d. |
n.a. |
3.0 x 10-1 |
n.a. |
|
Barium |
5.0 x 10-4 |
n.a. |
7.0 x 10-2 |
n.a. |
Alkalis (4) |
||||
|
Cadmium |
n.d. |
1.8 x 10-3 |
5.0 x 10-4 |
n.a. |
Sodium hydroxide |
n.d. |
n.a. |
n.d. |
n.a. |
|
Chromium (VI) |
n.d. |
1.2 x 10-2 |
5.0 x 10-3 |
n.a. |
Cyanides |
||||
|
Copper |
n.d. |
n.a. |
3.7 x 10-2 |
n.a. |
Potassium cyanide |
n.d. |
n.a. |
5.0 x 10-2 |
n.a. |
|
Iron |
n.d. |
n.a. |
n.d. |
n.a. |
Sodium cyanide |
n.d. |
n.a. |
4.0 x 10-2 |
n.a. |
|
Lead |
Based on biokinetic uptake models |
Zinc cyanide |
n.d. |
n.a. |
5.0 x 10-2 |
n.a. |
|||
|
Manganese |
5.0 x 10-5 |
n.a. |
4.7 x 10-2 |
n.a. |
Mineral Acids (4) |
||||
|
Mercury (3) |
3.0 x 10-4 |
n.a. |
3.0 x 10-4 |
n.a. |
Hydrochloric acid |
2.0 x 10-2 |
n.a. |
n.d. |
n.a. |
|
Nickel |
n.d. |
n.a. |
2.0 x 10-2 |
n.a. |
Hydrofluoric, nitric, and sulfuric acids |
n.d. |
n.a. |
n.d. |
n.a. |
|
Selenium |
n.d |
n.a. |
5.0 x 10-3 |
n.a. |
Phosphoric acid |
1.0 x 10-2 |
n.a. |
n.d. |
n.a. |
Footnotes are listed at end of Table 2-2.
Table 2-2 (continued)
Published EPA Human Health Toxicity Values for Chemical
Substances
Potentially Emitted by Metal Finishing Processes
|
RfC |
unit risk |
RfDo |
SFo |
RfC |
unit risk |
RfDo |
SFo |
||
|
Chemical |
mg/m3 |
(mg/m3)-1 |
mg/kg/day |
(mg/kg/day)-1 |
Chemical |
mg/m3 |
(mg/m3)-1 |
mg/kg/day |
(mg/kg/day)-1 |
|
Organic Acids (4) |
Chlorobenzene |
2.0 x 10-2 |
n.a. |
2.0 x 10-2 |
n.a. |
||||
|
Acetic, citric, oxalic, and tartaric acids |
n.d. |
n.a. |
n.d. |
n.a. |
Chloroform |
n.d. |
2.3 x 10-5 |
1.0 x 10-2 |
6.1 x 10-3 |
|
Other Inorganics |
Cresol (Cresylic acid) |
n.d. |
n.a. |
n.d. |
n.a. |
||||
|
Fluoride |
n.d. |
n.a. |
6.0 x 10-2 |
n.a. |
Cyclohexanone |
n.d. |
n.a. |
5.0 x 100 |
n.a. |
|
Potassium nitrate (5) |
n.d. |
n.a. |
1.6 x 100 |
n.a. |
1,2-dichlorobenzene |
2.0 x 10-1 |
n.a. |
9.0 x 10-2 |
n.a. |
|
Sulfur dioxide |
n.d. |
n.a. |
n.a. |
n.a. |
Dichloromethane |
3.0 x 100 |
4.7 x 10-7 |
6.0 x 10-2 |
7.5 x 10-3 |
|
Chlorine |
n.d. |
n.a. |
1.0 x 10-1 |
n.a. |
2-ethoxyethanol |
2.0 x 10-1 |
n.a. |
4.0 x 10-1 |
n.a. |
|
Organics |
Ethyl acetate |
n.d. |
n.a. |
9.0 x 10-1 |
n.a. |
||||
|
Acetone |
n.d. |
n.a. |
1.0 x 10-1 |
n.a. |
Ethylbenzene |
1.0 x 100 |
n.a. |
1.0 x 10-1 |
n.a. |
|
Acetone cyanohydrin |
n.d. |
n.a. |
8.0 x 10-4 |
n.a. |
Ethyl ether |
n.d. |
n.a. |
2.0 x 10-1 |
n.a. |
|
Benzene |
6.0 x 10-3 |
8.3 x 10-6 |
n.d. |
2.9 x 10-2 |
Formaldehyde |
n.d. |
1.3 x 10-5 |
2.0 x 10-1 |
n.d. |
|
Carbon disulfide |
7.0 x 10-1 |
n.a. |
1.0 x 10-1 |
n.a. |
Glycols (6) |
n.d. |
n.a. |
2.0 x 100 |
n.a. |
|
Carbon tetrachloride |
2.0 x 10-3 |
1.5 x 10-5 |
7.0 x 10-4 |
1.3 x 10-1 |
Isobutanol |
n.d. |
n.a. |
3.0 x 10-1 |
n.a. |
Footnotes are listed at end of Table 2-2.
Table 2-2 (continued)
Published EPA Human Health Toxicity Values for Chemical
Substances
Potentially Emitted by Metal Finishing Processes
|
RfC |
unit risk |
RfDo |
SFo |
RfC |
unit risk |
RfDo |
SFo |
||
|
Chemical |
mg/m3 |
(mg/m3)-1 |
mg/kg/day |
(mg/kg/day)-1 |
Chemical |
mg/m3 |
(mg/m3)-1 |
mg/kg/day |
(mg/kg/day)-1 |
|
Kerosene |
n.d. |
n.a. |
n.d. |
n.a. |
Pyridine |
n.d. |
n.a. |
1.0 x 10-3 |
n.a. |
|
Methanol |
n.d. |
n.a. |
5.0 x 10-1 |
n.a. |
Tetrachloroethylene |
n.d. |
n.d. |
1.0 x 10-2 |
5.2 x 10-2 |
|
Methyl ethyl ketone |
1.0 x 100 |
n.a. |
6.0 x 10-1 |
n.a. |
Toluene |
4.0 x 10-1 |
n.a. |
2.0 x 10-1 |
n.a. |
|
Methyl isobutyl ketone |
8.0 x 10-2 |
n.a. |
8.0 x 10-2 |
n.a. |
1,2,4-trichlorobenzene |
2.0 x 10-1 |
n.a. |
1.0 x 10-2 |
n.a. |
|
Mineral oil |
n.d. |
n.a. |
n.d. |
n.a. |
Trichlorofluoromethane |
7.0 x 10-1 |
n.a. |
3.0 x 10-1 |
n.a. |
|
Naphtha |
n.d. |
n.a. |
n.d. |
n.a. |
1,1,2-trichloro-1,2,2-trifluoroethane (Freon-113) |
3.0 x 101 |
n.a. |
3.0 x 101 |
n.a. |
|
n-butyl alcohol |
n.d. |
n.a. |
1.0 x 10-1 |
n.a. |
1,1,1-trichloroethane |
n.d. |
n.a. |
3.5 x 10-2 |
n.a. |
|
Nitrobenzene |
2.0 x 10-3 |
n.a. |
5.0 x 10-4 |
n.a. |
1,1,2-trichloroethane |
n.d. |
1.6 x 10-5 |
4.0 x 10-3 |
5.7 x 10-2 |
|
2-nitropropane |
2.0 x 10-2 |
n.a. |
n.d. |
n.a. |
Trichloroethylene |
n.d. |
n.d. |
6.0 x 10-3 |
1.1 x 10-2 |
|
Phenol |
n.d. |
n.a. |
6.0 x 10-1 |
n.a. |
Xylene (7) |
n.d. |
n.a. |
2.0 x 100 |
n.a. |
Source: (EPA 1995b; 1997e) Because the source references are updated
periodically, values in table should be verified before using in
a risk assessment.
(1) Includes metals and metal compounds
(2) n.d. - not determined. EPA has not determined a reference
concentration, reference dose, or unit risk for this chemical.
n.a. - not applicable. Cancer slope factor or unit risk value
has not been derived for this chemical because there is a lack of
evidence that indicates that this
chemical is a carcinogen, because this chemical is not carcinogenic,
or because the chemical is not carcinogenic by the oral or inhalation
exposure route.
(3) RfDo is for mercuric chloride; RfC is for elemental
mercury.
(4) The primary hazard associated with most acids and alkalis
is corrosivity. The more volatile acids produce irritating vapors.
(5) Values for potassium nitrate are based on the toxicity of
the nitrate anion and are expressed as nitrate-nitrogen.
(6) Values in table are for ethylene glycol; values for other
glycols will vary.
(7) Value is for mixed xylenes.
Exposure assessment seeks to determine the intensity, frequency, and duration of actual or potential exposures to a chemical in the environment (Figure 2-2). To assess exposure, information about the concentrations of chemicals in air, soil, water, and food is needed along with information about how humans may be exposed to these media (through breathing, skin contact with water or soil, drinking of water or milk, or eating produce or other foods) to assess the level of exposure (Table 2-3). An individual’s exposure will vary over time because chemical concentrations in the environment vary (e.g., with distance from the emissions source or with time as the chemical is dispersed by physical processes or is degraded by biological, chemical or physical processes) and because an individual’s location relative to the facility varies over time. To accurately describe exposure for different groups of people, chemical concentrations in water, air, soil, and food must be measured or estimated in several directions and at various distances from a source of chemical emissions. Information about the variation in concentrations over time also increases the accuracy of the exposure assessment. This information may be obtained by measuring and analyzing emissions or, for outdoor air concentrations, by using environmental fate and transport models and/or air dispersion models (e.g., ISC2, an existing EPA model, or the Total Risk Integrated Methodology, which is currently in development) to establish the relationship between emissions and chemical concentrations in the environment.
Figure 2-2
Conceptual Exposure Model for Electroplating Process
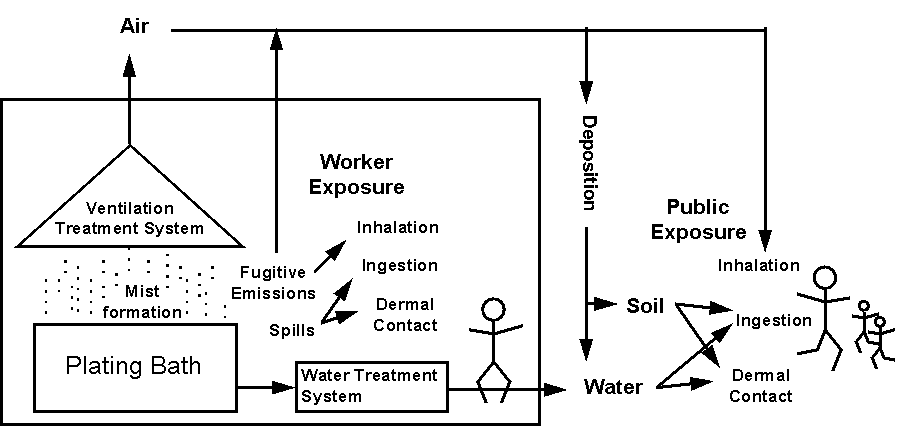
Concentration data for each medium are combined with information regarding population characteristics (e.g., volume of air breathed during different work activities, surface area of skin exposed to soil during gardening, volume of water drunk, or amount of food consumed) and
Table 2-3
Exposure Assessment Data Needs for Metal Finishing Facilities
|
Required Inputs |
|||
|
Exposed Population |
Chemical Concentration |
Exposure Duration and Frequency |
Population Characteristics |
|
Nearby Residents |
Site-specific and media-specific chemical concentration data (concentrations may be measured or modeled) Regional or local area chemical concentrations monitoring data Emissions rate information Local meteorology (wind direction, speed, turbulence, solar radiation) Surrounding terrain and buildings |
Duration of residence Daily activity patterns by age group: Hours spent at home Hours spent outdoors |
Age Sex Health Status |
|
Workers |
Site-specific and media-specific chemical concentration data (concentrations may be measured or modeled) Materials balance for facility processes Ventilation patterns and exchange rates Controls on indoor emissions |
Activity patterns by job description or category By job description or category: Hours worked per week Weeks worked per year Years worked per job Degree of exertion Engineering and administrative controls on exposure Personnel protective equipment |
Age Sex Health Status |
(Based on Schaum, 1997)
activity patterns (e.g., hours spent performing a specific work process, hours spent at home outdoors, number of years spent at a residential location) to determine the amount of chemical to which an individual is exposed (Table 2-4).
In Table 2-4, all values (exclusive of conversion factors) that are used to calculate the average daily dose have some degree of variability associated with them. Variability refers to observed differences attributable to heterogeneity in a population or exposure parameter. Sources of variability are the result of natural random processes and may be associated with
Table 2-4
Generalized Dose Equations
|
Route of Exposure |
Values |
|
Equation 2-1 Inhalation of Volatiles or Particulates ADDai = Ca x InhRa
x ETa x EFa x EDa |
ADDai = Average Daily Dose via air inhalation Ca = Chemical concentration in air (milligrams/meters3) InhRa = Inhalation rate (meters3/hour) ETa = Exposure time (hours/day) EFa = Exposure frequency (days/year) EDa = Exposure duration (years) BW = Body weight (kilograms) AT = Averaging time (years) |
|
Equation 2-2 Ingestion of Soil, Dust, or Surface Deposits ADDsi = Cs x IRs
x EFs x EDs x 10-6 |
ADDsi = Average Daily Dose via soil ingestion Cs = Chemical concentration in soil (milligrams/kilogram) IRs = Intake rate (milligrams/day) EFs = Exposure frequency (days/year) EDs = Exposure duration (years) 10-6 = Conversion factor (kilograms/milligram) BW = Body weight (kilograms) AT = Averaging time (years) |
|
Equation 2-3 Ingestion of Water ADDwater = Cw x IRw
x EFw x EDw |
ADDwi = Average Daily Dose via water ingestion Cw = Chemical concentration in water (milligrams/liter) IRw = Intake rate (liters/day) EFw = Exposure frequency (days/year) EDw = Exposure duration (years) BW = Body weight (kilograms) AT = Averaging time (years) |
Table 2-4 (continued)
Generalized Dose Equations
|
Equation 2-4 Dermal Contact with Soil, Dust or Surface Deposits ADDsc = Cs x SSAs
x AF x ABS x ETs x EFs x EDs
x 10-6 |
ADDsc = Average Daily Dose via soil contact Cs = Chemical concentration in soil (milligrams/kilogram) SSAs = Skin surface area (centimeters2/hr) AF = Soil adherence factor (milligram/centimeter2) ABS = Dermal absorption factor (unitless) ETs = Exposure time (hours/day) EFs = Exposure frequency (days/year) EDs = Exposure duration (years) 10-6 = Conversion factor (kilograms/milligram) BW = Body weight (kilograms) AT = Averaging time (years) |
|
Equation 2-5 Dermal Contact with Water ADDwc = Cw x Kp x
Tw x 10-3 x EVw x EFw
x EDw x SSAw
(steady state equation - see text for discussion of non-steady state approach) |
ADDwc = Average Daily Dose via water contact Cw = Chemical concentration in water (milligrams/liter) Kp = Dermal permeability constant (centimeters/hour) SSAw = Skin surface area (centimeters2) EVw = Event frequency (events/day) EFw = Exposure frequency (days/year) EDw = Exposure duration (years) 10-3 = Conversion factor (liters/centimeters3) BW = Body weight (kilograms) AT = Averaging time (years) |
environmental, lifestyle, and genetic differences among humans and other organisms. Examples of variability include physiological variation such as differences in body weight, breathing rate, and the amount of food and water consumed. Environmental variation may include fluctuations in air temperature, wind speed and direction, and soil conditions, all of which can affect the concentration of a chemical in a specific medium in the environment and thus the average concentration term that is used in the dose equation. Variability is usually not reducible by further measurement or study (although it can be better characterized).
Finally, different methods are used for inorganic and organic chemicals to calculate the amounts of these chemicals that are absorbed from water (EPA, 1992). The method for inorganic chemicals assumes a "steady-state" approach whereas the method for organic chemicals assumes a "nonsteady-state" approach. Steady-state means that the system reaches equilibrium over time and then does not change or changes only negligibly over the measurement time period. In general, the nonsteady-state approach is believed to most accurately reflect normal human exposure conditions since the short contact times associated with bathing and swimming generally mean that steady-state (equilibrium) conditions will not occur. The nonsteady-state method also accounts for the dose that can occur after the actual exposure event due to absorption of contaminants stored in fats and oils in the skin. Application of this method requires that the chemical in question partition between an organic solvent (octanol) and water. Inorganics do not exhibit this characteristic and thus the nonsteady-state methodology is not applicable to inorganics. The steady-state approach is therefore currently recommended for inorganics.
2.1.4 Risk CharacterizationRisk characterization combines the assessments of hazard, dose-response, and exposure to estimate the probability of specific harm to an exposed individual or population. It assesses the overall quality of the information in the assessment, identifying any sources of uncertainty associated with the risk assessment, and it indicates whether the assumptions made in the preceding steps tend to under- or over-estimate the level of risk.
Cancer risks are expressed as probabilities. As presented here the risk equations estimate the upper bound incremental increase in cancer risk over a lifetime due to the described exposure scenario. Because the calculated risks are upper bound estimates, the actual risks are unlikely to be greater. The calculated risks are the incremental increase over the "background" cancer rate among persons living in the U.S. The current U.S. background rate for all cancers over a lifetime is 30 about percent (i.e., 30 persons in 100 will be diagnosed with some form of cancer in their lifetime). The calculated risk due to the chemical exposure is the additional risk (e.g., one in a thousand, ten in a million, one in a million) above this lifetime background level.
For noncancer health effects the RfD or RfC is compared to the calculated dose or exposure concentration. When the exposure dose divided by the RfD or the exposure concentration divided by the RfC is greater than one (the calculated values are called "hazard quotients"), some potential for harmful health effects exists.
The general equations for the calculation of risk for carcinogens and hazard for noncarcinogens are as follows:
Risk via air inhalation
risk = unit risk*Ca*(InhRa/0.83)*(EFa/365)*(ETa/24)*(EDa/70)*(70/BW)2/3
HQ = Ca/RfC
Where:
Ca = Concentration in air
InhRa = Inhalation rate (meter3/hour)
EFa = Exposure frequency (days/year)
ETa = Exposure time (hours/day)
EDa = Exposure duration (years)
BW = Body weight (kilograms)
HQ = Hazard quotient
RfC = Reference concentration (may require adjusting from published
value to account for populations that differ from default EPA exposure
scenarios)
Risk via ingestion of soil
risk = oral slope factor*Cs*(IRs*EFs*EDs*CFs)/(BW*ATcarc)
HQ = [Cs*(IRs*EFs*EDs*CFs)/(BW*AT*365days/year)]/RfD
Where:
Cs = Concentration in soil
IRs = Intake rate for soil (milligrams/day)
EFs = Exposure frequency (days/year)
EDs = Exposure duration (years)
CFs = Conversion factor (10-6 kilograms/milligram)
BW = Body weight (kilograms)
ATcarc = Averaging time for carcinogens (25,550 days)
HQ = Hazard quotient
RfD = Reference dose
AT = Averaging time for noncarcinogens, equal to exposure duration
(years)
Risk via ingestion of water
risk = oral slope factor*Cw*(IRw*EFw*EDw)/(BW*ATcarc)
HQ = [Cw*(IRw*EFw*EDw)/(BW*AT)]/RfD
Where:
Cw = Concentration in water
IRw = Intake rate for water (liters/day)
EFw = Exposure frequency (days/year)
EDw = Exposure duration (years)
BW = Body weight (kilograms)
ATcarc = Averaging time for carcinogens (25,550 days)
HQ = Hazard quotient
RfD = Reference dose
AT = Averaging time for noncarcinogens, equal to exposure duration
(years)
Risk via dermal contact with water (steady-state conditions)
risk = oral slope factor*(Cw*Kp*Tw*CFw)*(EVw*EFw*EDw*SSAw)/(BW*ATcarc)
HQ = [(Cw*Kp*Tw*CFw)*(EVw*EFw*EDw*SSAw)/(BW*AT*365days/year)]/RfD
Where:
Cw = Concentration in water (milligrams/liter)
Kp = Dermal permeability (centimeters/hour)
Tw = Duration of event (hours/event)
CFw = Conversion factor (10-3 liters/centimeter3)
EVw = Event frequency (events/day)
EFw = Exposure frequency (days/year)
EDw = Exposure duration (years)
SSAw = Exposed skin surface area (centimeter2)
BW = Body weight (kilograms)
ATcarc = Averaging time for carcinogens (25,550 days)
HQ = Hazard quotient
RfD = Reference dose
AT = Averaging time for noncarcinogens, equal to exposure duration
(years)
Risk via dermal contact with soil/dirt
risk = oral slope factor* Cs*(SSAs*AFs*0.01*ETs*EFs*EDs*CFs)/(BW*ATcarc)
HQ = [Cs*(SSAs*AFs*0.01*ETs*EFs*EDs*CFs)/(BW*AT*365days/year)]/RfD
Where:
Cs = Concentration in water (milligrams/liter)
SSAs = Exposed skin surface area (meter2)
AFs = Soil adherence factor (unitless)
ETs = Exposure time (hours/day)
EFs = Exposure frequency (days/year)
EDs = Exposure duration (years)
CFs = Conversion factor (10-6 kilograms/milligram)
BW = Body weight (kilograms)
ATcarc = Averaging time for carcinogens (25,550 days)
HQ = Hazard quotient
RfD = Reference dose
AT = Averaging time for noncarcinogens, equal to exposure duration
Transfer of Chemicals Between Environmental Media. In addition to the sources of uncertainty regarding environmental concentrations, exposure factors, and toxicity values discussed above, the estimates of risk presented here do not account for the potential transfer of contaminants between different environmental media. For some chemicals and under some exposure conditions, the transfer of contaminants between environmental media may represent significant exposure pathways. For example, volatile contaminants in water may be released to the air during showering, dish washing, or other indoor household activities. The volatilized chemical could then be inhaled. A conceptual model of a facility should be prepared prior to performing a facility-specific risk assessment to identify those pathways of exposure that are important for that facility.
2.2 Assessing Risk For Chromium Electroplating Facilities
Chrome plating is a common process in metal finishing operations and as a result, chromium (primarily as hexavalent chromium) is prevalent in metal finishing emissions. Eight of the top 25 most commonly used metal finishing processes use chromium and it is estimated that about 2 million pounds per year of chromium are emitted to the air (23,000 pounds), discharged in wastewater (4,600 pounds), or disposed as wastewater sludge (900,000 pounds) or other solid waste (1,100,000 pounds) by hard chrome operations in the U.S. (EPA, 1995a). Hexavalent chromium is emitted during the chromium electroplating process in the form of a visible yellow mist. The mist is composed of entrained chromic acid droplets that form when hydrogen and oxygen gases are released from the surface of the plating solution.
Hexavalent chromium is a human carcinogen and can cause a variety of other adverse health effects (ATSDR, 1993; IARC, 1990; EPA, 1984). Breathing in chromium can cause irritation to the nose, such as runny nose, sneezing, itching, nosebleeds, ulcers, and, over long periods of time, holes in the nasal septum. Respiratory system effects (e.g., asthma) and immune system effects (e.g., allergic sensitivity from dermal exposure) have been documented (ATSDR, 1993).
2.2.2 Dose-Response Values for Hexavalent ChromiumInhalation Unit Risk. EPA estimates that the unit risk for hexavalent chromium is 0.012 (mg/m3)-1. The IRIS database provides the following information about the derivation of the inhalation unit risk.
"Results of occupational epidemiologic studies of chromium-exposed workers are found to be consistent across investigators and study populations. Dose-response relationships have been established for chromium exposure and lung cancer. Chromium-exposed workers are exposed to both chromium III and chromium VI compounds. Because only chromium VI has been found to be carcinogenic in animal studies, however, it was concluded that only chromium VI should be classified as a human carcinogen."
The IRIS database notes that the unit risk should not be used if the air concentration of hexavalent chromium exceeds 0.8 mg/m3. The assumption that the relationship between risk and concentration is linear may not be appropriate above this concentration (EPA, 1997e). Both higher and lower estimates of the inhalation unit risk for hexavalent chromium have been derived from human epidemiological studies (DTSC, 1994b; OSHA, 1995). A discussion of the merits and weaknesses of these various estimates is outside the scope of this paper, but is noted as a source of uncertainty in attempting to quantify human health risks.
Oral Slope Factor. The IRIS database does not contain an oral slope factor for hexavalent chromium because EPA believes that hexavalent chromium is not carcinogenic by the oral route of exposure. When ingested, hexavalent chromium is reduced to trivalent chromium in the saliva and gastric juice of the upper alimentary tract (Anderson et al., 1993 and references therein). Because the reduction of hexavalent chromium to trivalent chromium is relatively rapid and because trivalent chromium is not carcinogenic in animals EPA believes that ingested hexavalent chromium is not carcinogenic (EPA, 1991a). However, there is some disagreement with this conclusion and at least one state, California, has provisionally derived an oral slope factor of 4.2 x 10-1 for hexavalent chromium.
Reference Concentration. EPA does not currently list a RfC for hexavalent chromium. In 1991, EPA proposed a RfC of 0.002 mg/m3 for both hexavalent and trivalent chromium (EPA, 1991b), but this value has been withdrawn and the RfC for hexavalent and trivalent chromium are currently under review. It has been argued by Finley, et al. (1992) that separate RfCs should be established for the different valence states as well as for the different forms (particulates versus acidic mists) of chromium since they present different toxicological profiles. They proposed alternative RfCs of 1.2 and 0.12 mg/m3 for hexavalent chromium particulates and acidic mists, respectively.
Reference Dose. EPA’s RfD for soluble salts of hexavalent chromium, such as potassium and sodium dichromates and potassium and sodium chromates, is 0.005 mg/kg*day. This value was derived from a chronic (1-year) drinking water study in rats in which no adverse health effects were observed in the test animals over the treatment period. Similar "no-effect" levels have been observed in dogs and humans (EPA, 1997e). An uncertainty factor (margin of safety) of 500 was applied to the experimental "no-effect" dose in the rat study. The IRIS database notes:
"Confidence in the chosen study is low because of the small number of animals tested, the small number of parameters measured and the lack of toxic effect at the highest dose tested. Confidence in the data base is low because the supporting studies are of equally low quality, and teratogenic and reproductive endpoints are not well studied. Low confidence in the RfD follows."
Dermal Toxicity Values. Because there are few toxicity data for chemicals administered to the skin of laboratory animals or humans, toxicity via dermal exposure is often evaluated using oral RfDs or slope factors (EPA, 1992). This introduces a degree of uncertainty in risk estimates because chemicals introduced via the oral route may behave differently than if introduced through the skin. Since pharmacokinetic data are not available for most chemicals to help interpret or correct for potential differences in chemical behavior/toxicity, it is often uncertain how the use of oral toxicity factors may affect the estimate of true risk from dermal exposure. The oral RfD for hexavalent chromium is used to assess the potential noncarcinogenic risk that can result from dermal exposure.
2.2.3 Exposure AssessmentTwo worker and two residential exposure scenarios that include possible exposures via inhalation, ingestion of drinking water, incidental ingestion of soil or other dirt (e.g., through contamination of hands and subsequent hand to mouth actions), and dermal contact are evaluated (Figures 2-3 and 2-4). Two levels of hexavalent chromium concentrations were selected for each medium in the example risk assessments from among values reported in the scientific literature (Table 2-5).
Table 2-5
Environmental Concentrations of Hexavalent Chromium
Used in the
Example Risk Assessment
|
Environmental Medium |
||||
|
Air |
Water |
Soil/Dirt |
||
|
Workers |
Residents |
Workers/Residents |
Workers/Residents |
|
|
Concentration Level 1 |
0.5 mg/m3 |
1 ng/m3 |
0.02 mg/L |
0.4 mg/kg |
|
Concentration Level 2 |
5 mg/m3 |
5 ng/m3 |
0.2 mg/L |
4.0 mg/kg |
Although some professional judgment was used to estimate worker exposures and "typical" residential exposures, data used in the example risk assessment for an electroplating facility are based generally on population information compiled by EPA (EPA, 1989). The values used in the calculation of risk estimates (Section 2.2.4) are summarized in Table 2-6. These values represent only a few of the possible exposure scenarios. Actual exposures would vary among individual facilities. For the dermal exposure pathway a dermal permeability factor, Kp, is needed to determine exposure dose. A Kp of 2.0 x 10-3 cm/hr, the recommended value for sodium chromate, is used (EPA, 1992). This value was selected by EPA because it was reported in several studies using both human and animal subjects. Experimentally-derived Kp values ranging from 3.1 x 10-4 to 1.2 x 10-3 cm/hr have been reported for other chromium compounds.
Concentrations in Workplace Air. OSHA’s Integrated Management Information System (IMIS) database contains the results of 424 personal, full-shift air samples that were collected between 1979 and 1993 and were analyzed for hexavalent chromium (Table 2-7). These samples were collected in industry sectors classified in SIC Codes 33 through 39 and represent 8-hour TWA exposure of employees with job titles such as "plater," "plating operator," "electroplater," and "anodizer." Almost two-thirds of the IMIS samples were obtained in industry sectors within SIC Code 3471. Approximately 92% of the values were less than 10 mg/m3 and almost 75% were less than 1.0 mg/m3. OSHA is currently considering a new workplace exposure limit for hexavalent chromium in the range of 0.5 to 5.0 mg/m3 (Freeman and Condit, 1995). Workplace concentrations of 0.5 mg/m3 and 5.0 mg/m3 are used in the example risk assessment calculations.
Table 2-6
Summary of Exposure Factors Used in Example Risk Assessment
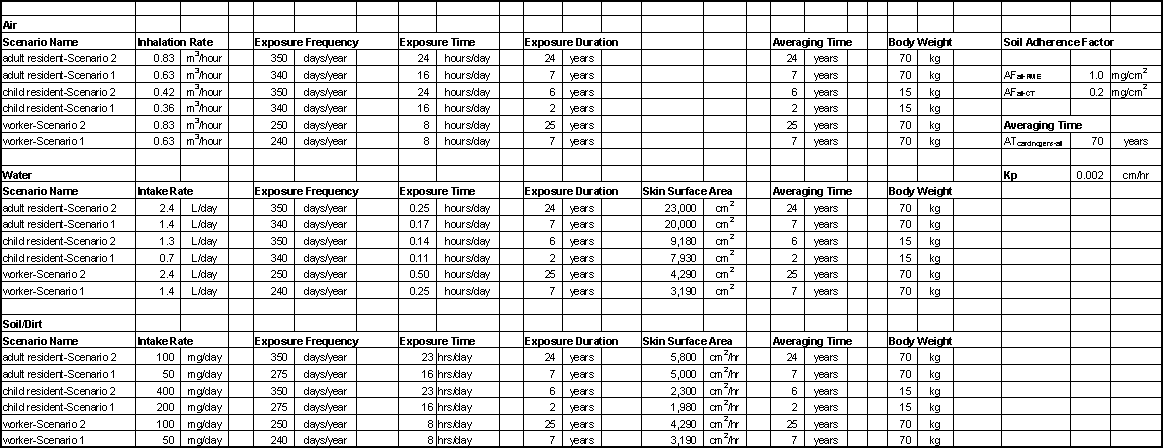
Note that the higher concentration exceeds the recommended upper concentration limit for use with EPA’s unit risk as described in Section 2.2.2.
Table 2-7
Relative Proportion of Hexavalent Chromium Concentrations
Measured in the Workplace
|
Concentration in Air (mg/m3) |
||||||
|
< 0.1 |
0.1 - 1.0 |
1.0 - 2.0 |
2.0 - 5.0 |
5.0 - 10.0 |
10.0 - 50.0 |
> 50.0 |
|
70% |
3.5% |
2.8% |
8.5% |
6.8% |
6.4% |
2.6% |
Total does not equal 100% due to rounding.
Concentrations in Ambient Air. Measurements of atmospheric hexavalent chromium in a non-industrial area of New Jersey ranged from 0.2 to 3.8 nanograms (ng)/m3, with a mean of 1.2 ng/m3 (Finley, et al., 1995). Measurements in 20 California cities reportedly ranged from less than 0.2 to 9 ng/m3 with a majority of samples at about 1 ng/m3 (Finley, et al., 1996). A recent Canadian study reported atmospheric concentrations of 0.1 to 1.6 ng/m3, with a geometric mean of 0.55 ng/m3 (Bell and Hipfner, 1997). The Canadian study also cites other works that indicate a hexavalent chromium concentration in the range of 1 to 5 ng/m3 in urban areas. Ambient air concentrations of 1 and 5 ng/m3 are used in the example risk assessment calculations for the residential exposure scenario. These concentrations represent the estimated current background concentration of hexavalent chromium from natural and anthropogenic (human-related) sources, which may include some contribution of chromium from plating facilities. Concentrations of hexavalent chromium may be higher than background in the vicinity of chromium plating facilities. Any increase in concentration that results from emissions would result in an incremental increase in risk for exposed populations. As noted above, air concentrations can be determined by dispersion modeling or by measurement.
Concentrations in Drinking Water. A survey of tap water in the United States conducted during the 1970s found that the concentration of total chromium ranged from 0.4 to 8.0 mg/L, with a mean of 1.8 mg/L (ATSDR, 1993). ATSDR notes that these values may be higher than the actual values, due to inadequate flushing of tap water before sample collection. In addition, because the values are for total chromium, hexavalent chromium would be expected to be some percentage of the total chromium concentration. Hexavalent chromium concentrations of 0.02 and 0.2 mg/L, approximately 1% and 10% of the mean total chromium concentration in the cited study, are used in the example risk assessment calculations for both the worker and residential exposure scenarios.
Concentrations in Soils. The natural chromium concentrations in soils vary greatly and depend on the composition of the parent rock from which the soils were formed. The trivalent form of chromium predominates in most soils (ATSDR, 1993). A study of soils in the United States by the United States Geologic Survey (USGS, 1984) reported that the concentration of total chromium ranged from 1 to 2,000 mg/kg, with a geometric mean of 37 mg/kg. A Canadian
Figure 2-3
Exposure Scenarios for Workers
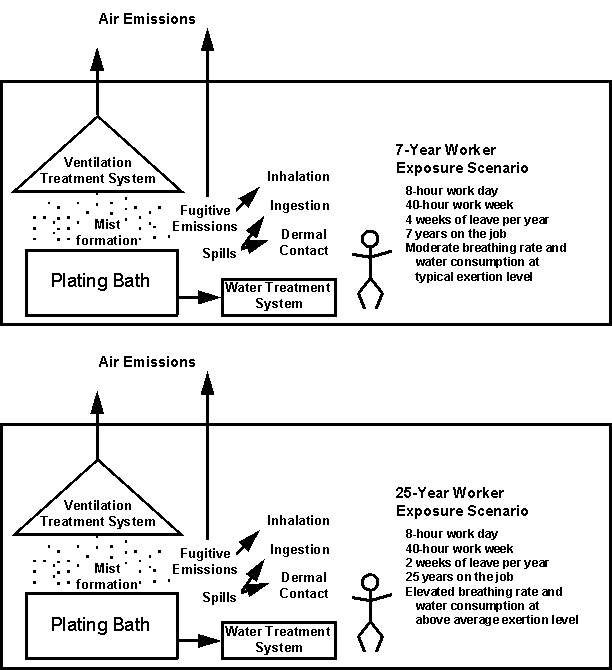
Figure 2-4
Exposure Scenarios for Residents
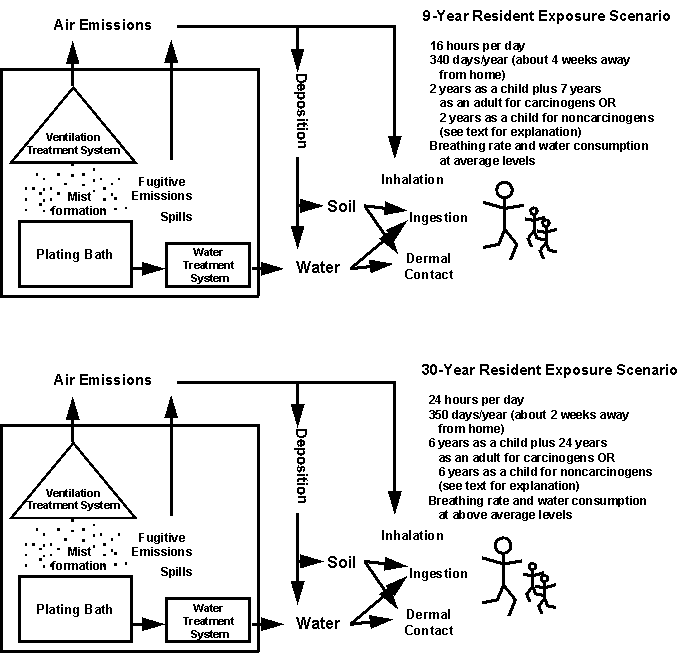
study reported concentrations that ranged from 5 to 1,500 mg/kg, with a mean of 43 mg/kg (ATSDR, 1993). Hexavalent chromium concentrations of 0.4 and 4 mg/kg, approximately 1% and 10% of the mean total chromium concentration in the two cited studies, are used in the example risk assessment calculations for both the worker and residential exposure scenarios.
The estimate of workplace exposure via dermal pathways contains a great deal of uncertainty because concentrations of hexavalent chromium in the work environment are highly dependent upon the effectiveness of industrial hygiene practices applied in the workplace. While concentrations of chromium on surfaces near electroplating tanks may be high, the use of personnel protective equipment such as gloves would greatly reduce exposure via dermal contact.
2.2.4 Risk CharacterizationUsing the input variables for toxicity, exposure and environmental concentrations described in the preceding sections, estimated excess cancer risks and hazard quotients have been calculated for exposure to hexavalent chromium in two settings, a workplace exposure scenario and a residential exposure scenario. For each of the two exposure settings the exposure factors (e.g., inhalation rates, exposure time, exposure duration) and environmental concentrations of hexavalent chromium were varied to yield a total of four estimates of cancer risk and assessments of the potential for adverse health effects due to noncarcinogenic effects. Estimates of excess cancer risks to residents were calculated using the assumption that exposure occurred over a period of time that includes both adult and childhood exposure. Hazard quotients for residents were calculated using exposure factors for children only. Hazard quotients for children are higher than for adults (at exposure to equivalent environmental concentrations) because children have lower body weights and higher intakes via certain pathways (e.g., ingestion of soil) than adults. Calculations were performed by entering example exposure data (Section 2.1.3), example environmental concentrations (Section 2.1.4), and risk equations (Section 2.1.4) onto a computer-based spreadsheet in Microsoft® Excel.
Residential Exposure Scenario. The four estimates of total lifetime excess cancer risk (Table 2-8 and Figure 2-5) for residential exposures are calculated for hypothetical 9- and 30- year exposures (Table 2-5) at the lower and upper environmental concentrations (Table 2-6). The 9-year exposure assumes that the exposed individual is present for 2 years as a child and 7 years as an adult. The 30-year exposure assumes that the exposed individual is present for 6 years as a child and 24 years as an adult. The scenarios yield estimates of total lifetime excess cancer risks that range from 8.3 x 10-7 to 2.7 x 10-5. All of this risk is derived from the inhalation pathway because hexavalent chromium is not believed to be carcinogenic by ingestion or dermal uptake. Because the risk calculation for the inhalation pathway uses a hexavalent chromium concentration in air that has been reported for several urban areas, these values reflect a minimum (background) estimate of risk for the inhalation route of exposure that can be calculated using this methodology and the exposure factors presented herein.
The hazard quotients for the residential exposures are all well below unity, indicating that concentrations of hexavalent chromium for these exposure scenarios would not present a noncancer health hazard.
Table 2-8
Predicted Lifetime Excess Cancer Risks and Hazard
Quotients
for Exposure to Hexavalent Chromium
Excess Cancer Risks
|
Worker Exposure Scenario |
Resident Exposure Scenario |
|||
|
7-Year Exposure |
25-Year Exposure |
9-Year Exposure |
30-Year Exposure |
|
|
Concentration Level 1 |
2.0 x 10-4 |
4.3 x 10-3 |
8.3 x 10-7 |
5.3 x 10-6 |
|
Concentration Level 2 |
* |
* |
4.1 x 10-6 |
2.7 x 10-5 |
* Concentration of hexavalent chromium exceeds valid range for calculating risk using EPA’s unit risk value.
Hazard Quotients for Noncarcinogens
|
Worker Exposure Scenario |
Resident Child Exposure Scenario |
|||
|
7-Year Exposure |
25-Year Exposure |
2-Year Exposure |
6-Year Exposure |
|
|
Concentration Level 1 |
< .001 |
< .001 |
.001 |
.003 |
|
Concentration Level 2 |
.001 |
.004 |
.012 |
.029 |
There is uncertainty associated with the environmental concentrations of hexavalent chromium in water and soil. While several estimates of hexavalent chromium concentrations in air were available in the scientific literature, the values for water and soil are based on percentages of measured values for total chromium. As actual concentrations may be higher or lower, it is uncertain how the use of these estimates may affect the estimate of risk from oral and dermal exposures. Direct measurement of facility-specific concentrations would reduce the uncertainty associated with exposure point concentrations.
Worker Exposure Scenario. Two estimates of total lifetime excess cancer risk for workplace exposures are calculated for hypothetical 7- and 25-year worker exposures at the lower environmental concentration. No excess cancer risk is included for the highest workplace air concentration because it exceeds 0.8 mg/m3, the maximum concentration for which EPA considers its unit risk to be valid. The scenarios yield estimates of lifetime excess cancer risks that range from 2.0 x 10-4 to 4.3 x 10-3. As is the case for the residential exposure, all of this risk is derived from the inhalation pathway.
Although the workplace estimates of risk are higher than that usually associated with environmental exposures (EPA generally regulates carcinogens in the range of one in ten thousand [1 x 10-4] to one in a million [1 x 10-6] chances of excess cancer risk), the estimated risks are similar to the one in a thousand (1 x 10-3) risk level that OSHA considers a "significant" risk when making risk management decisions to regulate workplace carcinogens. However, OSHA’s estimates of risk are not directly comparable to these estimates because OSHA assumes an 8-hour day and a 240-day work-year over a 45-year working lifetime and because OSHA develops its own cancer potency factors, which may differ from EPA’s (Rhomberg, 1996). Actual workplace concentrations and exposure factors would vary among facilities and would depend upon many factors including the operation and maintenance of ventilation systems, administrative controls to limit worker exposures, and the use of personnel protective equipment. Direct measurement of chromium concentrations within workers’ breathing zones would reduce uncertainty associated with worker exposures.
Figure 2-5
Lifetime Excess Cancer Risk for Community Residents
for Example Risk Assessment
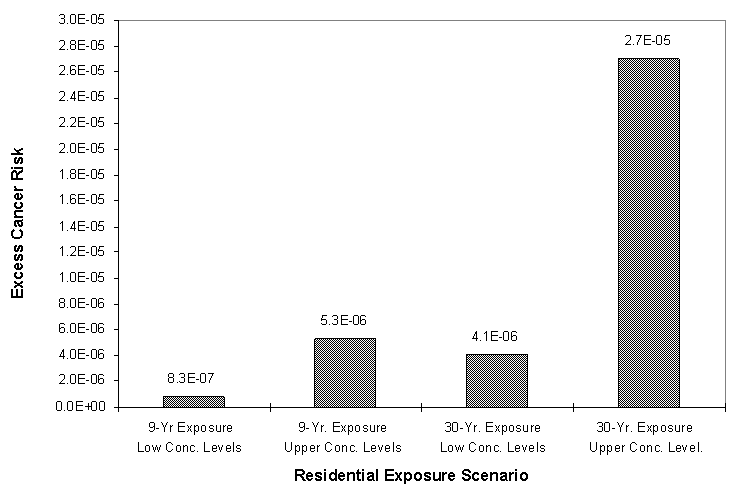
Risk assessments should be prepared with potential risk managers and stakeholders in mind, to assure that appropriate information will be provided in a format that is understandable and useable to all interested parties. An appropriate level of detail for data presentation and reports should be identified early in the process because potential risk managers are a diverse group that may have varying degrees of technical expertise. Presenting risk assessment results in a clear and concise format increases the likelihood that risk managers will remain engaged in the process and will contribute input on political, social, and economic issues that are part of the risk management process.
This paper presents example exposure scenarios and identifies the data inputs (i.e., environmental concentrations, measures of exposure, and toxicity information) that are needed to assess the potential health risks to workers and residential populations that are exposed to chemical emissions from metal finishing facilities. Such risk-based information is desired by stakeholders within EPA’s CSI Metal Finishing Sector but has not been made widely available to them. While some emissions data for metal finishing facilities are available, translation of that information into statements about potential health effects of those emissions has been limited. Risk-based information is needed to assist risk managers and stakeholders in identifying important risks, so that they may prioritize those risks and allocate resources to address them. Success of the risk assessment approach in meeting the need of risk managers to understand the health risks associated with facility emissions requires that risk managers be informed about the risk assessment process. The elucidation of the methods of risk assessment as described herein is an important starting point for the necessary dialog between risk managers and stakeholders involved with the Metal Finishing Sector, and the risk assessor.
3. Summary
Facility-based risk characterization for workers and surrounding communities is a high priority issue for stakeholders in EPA’s CSI Metal Finishing Sector. Platers, environmental groups, community groups, labor, and regulators all need and want to know what emissions are coming out and in what amounts from metal finishing operations (EPA, 1997a). They also want to know what health risks those emissions create for workers and the surrounding communities. Potential health risks from emissions can be described and quantified by the process of risk assessment. Risk assessment evaluates information on the hazardous properties of chemicals and the extent of human exposure, and characterizes the resulting risks. EPA and others use a risk assessment process formalized by the National Academy of Sciences that is comprised of four steps--hazard identification, dose-response assessment, exposure assessment, and risk characterization (NRC, 1983; 1994).
The Research and Technology Work Group of the CSI Metal Finishing Subcommittee identified the development and application of simple methods to characterize the emissions from plating operations as a high priority item in its R&D Plan (EPA, 1997a). Specifically, the R&D Plan recommended characterizing the emissions from plating operations and from them the risks to workers, surrounding communities, and the environment. The objectives of this project were to address the recommendation of the R&D Plan by 1) identifying the types and sources of information needed to assess risks to workers and surrounding communities from metal finishing facilities (hazard identification and dose-response assessment), 2) developing a general facility model that describes potential human exposure pathways (exposure assessment), 3) presenting equations that characterize the exposure pathways from emission sources to workers and the public, and 4) quantifying the lifetime excess cancer risk and potential for other health hazards from hexavalent chromium in a screening risk assessment process (risk characterization).
By explaining the steps performed and the data needed to conduct a risk assessment it is hoped that this paper will assist those associated with the industry to better understand the risk assessment process and the questions that can be answered by the process. Based on the work reported here, it is concluded that the general methodology for carrying out risk assessments for metal finishing workers and surrounding communities is known; that it is possible to calculate such risks for a number of worker and community scenarios, and that the methodology has limitations associated with toxicity information for chemicals used in the metal finishing sector and with exposure inputs, such as environmental concentrations of chemicals and activity patterns of potentially exposed individuals.
Important needs for additional development of this approach to facility-based risk characterization for hard chromium plating and other operations are:
- communication between risk managers, stakeholders, and risk assessors to identify issues of greatest importance to end users of the risk assessment information;
- determination of environmental concentrations of chemical emissions of interest (either modeled or measured) for use in risk assessments; and
- refinement of exposure information for potentially exposed populations.
4. REFERENCES
Anderson, R.A., T. Colton, J. Doull, J.G. Marks, and R.G. Smith, 1993. Designing a biological monitoring program to assess community exposure to chromium: conclusions of an expert panel. Journal of Toxicology and Environmental Health 40:555-583.
ATSDR, 1993. Toxicological Profile for Chromium. Prepared by Syracuse Research Corporation under subcontract to Clement International Corporation for the Agency for Toxic Substances and Disease Registry, Public Health Service, United States Department of Health and Human Services.
Bell, R.W., and J.C. Hipfner, 1997. Airborne hexavalent chromium in southwestern Ontario. J. Air & Waste Management 47:905-910.
Browner, Carol M., 1994. Speech delivered by Carol M. Browner, Administrator, United States Environmental Protection Agency, at the Center for National Policy Newsmaker Luncheon, Washington, D.C. July 20.
CAMP, 1995. Profile for the Metal Finishing Industry-Draft. Prepared for the Cleveland Advanced Manufacturing Program (CAMP) under contract to the Energy Environment and Manufacturing project of the Technology Reinvestment Program (EEM-TRP). Waste Reduction Institute for Training and Applications Research (WRITAR).
DTSC (California Department of Toxic Substances Control), 1994a. Preliminary Endangerment Assessment Guidance, January.
DTSC, 1994b. California Cancer Potency Factors, California Department of Toxic Substances Control, Standards and Criteria Work Group. 1 November.
EPA, 1984. Health Assessment Document for Chromium. Final Report. EPA-600/8-83-014F. Environmental Criteria and Assessment Office, Research Triangle Park, North Carolina. NTIS PB85-115905.
EPA, 1986. Guidelines for Carcinogen Risk Assessment. 51 Federal Register 33992-34003.
EPA, 1989. Exposure Factors Handbook. United States Environmental Protection Agency, Office of Research and Development. EPA/600/8-89/043. May (a preliminary draft revision of this document was released for comment in August 1996).
EPA, 1991a. National Primary Drinking Water Regulations, Final Rule. Code of Federal Regulations 40: Parts 141,142, and 143, January 30.
EPA, 1991b. Health Effects Assessment Summary Tables, FY-1991. Publ. No. OERR 9200, 6-303 (91-1) United States Environmental Protection Agency, Office of Emergency and Remedial Response, Washington, D.C.
EPA, 1992. Dermal Exposure Assessment: Principles and Applications, Interim Report. United States Environmental Protection Agency, Office of Research and Development. EPA/600/8-91/011B.
EPA, 1993. Introduction to IRIS. August 18.
EPA, 1994. A New Generation of Environmental Protection. CSI: Administrator’s Update, Number 12. July 29.
EPA, 1995a. Draft Report on Emissions from Metal Finishing Operations. United States Environmental Protection Agency, Office of Research and Development. March 31.
EPA, 1995b. Health Effects Assessment Summary Tables. United States Environmental Protection Agency, Office of Emergency and Remedial Response, Washington, D.C. May.
EPA, 1995c. Profile of the Fabricated Metal Products Industry. Office of Compliance Sector Notebook Project, United States Environmental Protection Agency, Office of Enforcement and Compliance Assurance. September.
EPA, 1995d. Pollution Prevention Assessment for a Manufacturer of Electroplated Truck Bumpers. United States Environmental Protection Agency, National Risk Management Research Laboratory. September.
EPA, 1996a. Proposed Guidelines for Carcinogen Risk Assessment, 61 Federal Register 17960, April 23.
EPA, 1996b. Proposed Guidelines for Ecological Risk Assessment, 61 Federal Register 47552, September 9.
EPA, 1997a. National Metal Finishing Environmental R&D Plan. Common Sense Initiative Metal Finishing Subcommittee. January 7.
EPA, 1997b. Discussion Draft, Strategic Goals Program, Part 1: National Performance Goals, Part 2: Action Plan. Common Sense Initiative, Metal Finishing Sector. July 15.
EPA, 1997c. 1995 Toxics Release Inventory Public Data Release. United States Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances. EPA 745-R-97-005. April.
EPA, 1997d. Common Sense Initiative. Metal Finishing Sector Fact Sheet. United States Environmental Protection Agency. EPA 742-B-96-007. March.
EPA, 1997e. Integrated Risk Information System. National Center for Environmental Assessment. Downloaded from National Library of Medicine on-line service. July.
Finley, B.L., D.M. Proctor, and D.J. Paustenbach, 1992. An alternative to EPA’s proposed inhalation reference concentrations for hexavalent and trivalent chromium. Regulatory Toxicology and Pharmacology 16:161-176.
Finley, B.L., B.D. Kerger, D.G. Dodge, S.M. Meyers, R.O. Richter, and D.J. Paustenbach, 1996. Assessment of airborne hexavalent chromium in the home following use of contaminated tapwater. J. Exposure Analysis and Environ. Epidemiol. 6:229-245.
Freeman, C., and K. Condit, 1995. The new OSHA regulations for hexavalent chromium. 16th AESF/EPA Pollution Prevention & Control Conference Proceedings. 13-15 February.
IARC (International Agency for Research on Cancer, 1990. Chromium and Chromium Compounds. IARC Monograph Series, World Health Organization, Volume 49.
Klaasen, C.D., 1995. Casarett and Doull’s Toxicology, The Basic Science of Poisons, McGraw-Hill, Health Publications Division, New York.
Murphy, M., (ed.), 1996. Metal Finishing: 64th Guidebook and Directory Issue, Vol. 94, No. 1A. Elsevier, New York.
NCMS (National Center for Manufacturing Sciences), 1994. Pollution Prevention and Control Technology for Plating Operations. Prepared for the National Center for Manufacturing Sciences and the National Association of Metal Finishers, George Cushnie.
NRC (National Research Council), 1983. Risk Assessment in the Federal Government: Managing the Process. National Academy Press, Washington, D.C.
NRC, 1994. Science and Judgment in Risk Assessment. National Academy Press, Washington, D.C.
OSHA (Occupational Safety and Health Administration), 1995. Evaluation of Epidemiological Data and Risk Assessment for Hexavalent Chromium. Prepared for OSHA by K.S. Crump Division, ICF Kaiser. OSHA Hexavalent Chromium Docket, Docket H-054A, Exhibit #13-5.
Presidential/Congressional Commission on Risk Assessment and Risk Management, 1997a. Final Report, Volume 1, Framework for Environmental Health Risk Management.
Presidential/Congressional Commission on Risk Assessment and Risk Management, 1997b. Final Report, Volume 2, Risk Assessment and Risk Management in Regulatory Decision-Making.
Puri, I.K., 1993. The Metal Products and Machinery Industry - Issues for Pollution Reduction. Produced in cooperation with Paul Shapiro, United States Environmental Protection Agency, Office of Environmental Engineering and Technology Demonstration through an American Association for the Advancement of Science Summer Environmental Science and Engineering Fellowship. August 9.
Rhomberg, L.R., 1996. A survey of methods for chemical health risk assessment among federal regulatory agencies, prepared at the request of the Presidential/Congressional Commission on Risk Assessment and Risk Management.
Schaum, J., 1997. Personal communication with John Schaum, National Center for Environmental Assessment, United States Environmental Protection Agency.
Thistle Publishing, 1996. Risk Assistantä, version 1.1, Alexandria, VA.
USGS (United States Geological Survey), 1984. Element Concentrations in Soils and Other Surficial Materials of the Conterminous United States. USGS Professional Paper 1270.
ACRONYMS
AAAS = American Association for the Advancement
of Science
ABS = dermal absorption factor
AF = soil adherence factor
ADD = average daily dose
AESF = The American Electroplaters and Surface Finisher's Society,
Inc.
AMSA = Association of Municipal Sewerage Agencies
AT = averaging time
ATSDR = Agency for Toxic Substances and Disease Registry
BW = body weight
Ca = concentration in air
Cs = concentration in soil
Cw = concentration in water
CAMP = Cleveland Advanced Manufacturing Program
carc = carcinogen
cm = centimeter
CSI = Common Sense Initiative
DTSC = Department of Toxic Substances Control (California)
ED = exposure duration
EF = exposure frequency
EPA = Environmental Protection Agency (US)
ET = exposure time
EV = event frequency
HQ = Hazard quotient
hr = hour
IARC = International Agency for Research on Cancer
IMIS = Integrated Management Information System
IR = intake rate
InhR = inhalation rate
IRIS = Integrated Risk Information System
kg = kilogram
Kp = dermal permeability constant
L = liter
LOAEL = lowest-observed-adverse-effect-level
m = meter
m3 = cubic meters
mg = milligram
m
g = microgramNOAEL = no-observed-adverse-effect-level
NCEA = National Center for Environmental Assessment
NCMS = National Center for Manufacturing Sciences
ng = nanogram
NRC = National Research Council
NRDC = Natural Resources Defense Council
ORD = Office of Research and Development
OSHA = Occupational Safety and Health Administration
R&D = Research and Development
RfC = Reference concentration
RfD = Reference
SF = Cancer slope factor
SIC = Standard Industry Classification
SSA = skin surface area
TWA = time-weighted average
Tw = duration of event
UAW = United Auto Workers
USGS = United States Geological Survey
APPENDIX A
Output from Risk Assistantä
RISK*ASSISTANT for Windows Report
R*A Standard Report 09/28/97
13:18
Approach
The procedures used by RISK*ASSISTANT to calculate exposures have been reviewed by the Office of Health and Environmental Assessment of the U.S. EPA.Default parameters for calculating exposures have been extracted from these U.S.EPA documents:
U.S.EPA, Office of Solid Waste and Emergency Response,Risk Assessment Guidance for Superfund,Volume I: Human Health Evaluation Manual,Supplemental Guidance: Standard Default Exposure Factors. Directive 9285.6-03; Interim Final. March 25, 1991.
J.Konz, K.Lisi, and E. Friebele, Exposure Factors HandbookU.S.EPA, Office of Health and EnvironmentalAssessment, EPA/600/8-89/043; March 1989.
Aggregation Method Used in Analysis
The following table lists the technique used to combine data from multiple samples (i.e. the Aggregation Method) for each environmental medium included in the analysis. For each class of data qualifier that might apply to the sample set (non-detects, estimated values, controls not within limits, or concentration estimated at a dilution factor), the approach used to assign a concentration to the qualified data values is presented.
Contaminated Aggregation Treatment of Qualified Samples
Medium Method NonDetect Estimated Ctls not within limitsDilution Factor
(U) (J) (R,B,E,M,N,W,*) (D)
Four options are available for dealing with qualified sample data (i.e. concentration values for which a proxy value has been entered, accompanied by one of the four classes of data qualifiers recognized by RISK*ASSISTANT.A separate decision can be made for each class of qualified data. The user may either use all proxy values as entered, use one-half of the entered proxy value, exclude (drop) the qualified data, or set the concentration for the qualified data to zero.
Chemical Concentrations in Contaminated Media
The concentration values presented in this table are expressed using the S.I. (Systeme Internationale, also called metric) units most commonly employed in risk assessment. They may differ from the units used in data entry.
CAS# Chemical Name
540-29-9 CHROMIUM (VI)
GW SW Air Soil Sed Veget Fruit Fish Dairy Meat
ug/l ug/l ug/cu m mg/kg mg/kg ug/kg ug/kg ug/kg ug/kg ug/kg
0.2 0.001 0.4
NOTE: scientific notation is used for numbers less that 0.000001
and greater than 1000000.
For example:0.00000021 = 2.1e-7 = 2.1 / 10000000 and 21000000 =
2.1e7 = 2.1 * 10000000.
GW = Groundwater, SW = Surface Water, Sed = Sediment, Veget = Vegetable.
The listed concentration in each medium for a chemical reflects the selection of sample values employed in aggregation, the aggregation method selected, and the approach used for dealing with qualified data. Where concentration data were entered directly, the assessor should be prepared to explain the values that were chosen.
Exposure Scenarios
In RISK*ASSISTANT,every exposure scenario is associated with a single contaminated medium. While some scenarios potentially apply to more than one medium, any individual assessment must assign a scenario to only one contaminated medium.
Groundwater
Drinking Water
Air
Indoor Air
Outdoor Air
Soil
Dust/Soil Indoors
Dust/Soil Outdoors
The dose and concentration estimates in this assessment, as well as any risk estimates that are derived from them, refer only to the specific exposures that have been described. This description consists of:
- Contaminant concentrations in one or more environmental media.
- For each contaminated medium, one or more scenarios describing
how a person contacts that medium.
- Parameters that describe each scenario, both in general, and for
each potential route of exposure (oral, inhalation, or dermal).
An assessment that incorporates other exposures, or that does not incorporate all of the exposures described in this analysis, will yield different results. This list presents the exposure scenarios evaluated for each contaminated medium considered in this assessment.
Cross-Media Transfer Equations Used to Generate Exposure Estimates
For some exposure scenarios a contaminant concentration specified in one environmental medium must be converted to a concentration in another medium, to which a person is exposed. (For example, in order to evaluate inhalation exposures while showering, contaminant concentrations in domestic water must be converted to concentrations in bathroom air.) The following equations were used in this assessment to predict such cross-media contaminant transfers in each of the indicated exposure scenarios. INHALATION OF PARTICULATES INSIDE THE RESIDENCE - Soil to respirable Particulates
REFERENCES:
(1) Wark, K. & Warner, C.F.Air Pollution: Its Origin and Control,Second
Ed., New York: Harper & Row, 1981.
(2) Hawley, J.K. 'Assessment of Health Risk from Exposure to Contaminated
Soil.' Risk Analysis, 5,(1985)289.
EQUATION: C(i) = D * R * f * C(s)
PARAMETERS User Value
C(i) Inhaled Concentration of Contaminant Calculated
C(s) Concentration in Soil Chemical Specific
R = Respirable Fraction of Dust 73.00%
f = Proportion of Contaminated Dust 0.80%
D = Dust Concentration 56.00 ug per cu.m
INHALATION OF PARTICULATES OUTSIDE THE RESIDENCE - Soil or Sediment to respirable
Particulates
REFERENCES: Wark, K. & Warner, C.F.Air Pollution: Its Origin
and Control,Second Ed.,
New York: Harper & Row, 1981.
EQUATION: C(i) = D * R * f * C(s)
PARAMETERS User Value
C(i) Inhaled Concentration of Contaminant Calculated
C(s) Concentration in Soil Chemical Specific
R = Respirable Fraction of Dust 73.00%
f = Proportion of Contaminated Dust 1.00%
D = Dust Concentration 75.00 ug per cu.m
Concentrations in Media after Transfers
For some exposure scenarios a contaminant concentration specified in one environmental medium must be converted to a concentration in another medium, to which a person is exposed. For example, in order to evaluate inhalation exposures while showering, contaminant concentrations in domestic water must be converted to concentrations in bathroom air. The values presented in this table are concentrations of contaminants in exposure media that have been predicted for specific exposure scenarios from concentrations that were specified in other media.
Chemical Name
Contaminated Media / Scenario Calculated Concentrations
GW SW Air Soil Sed Veget Fruit Fish Dairy Meat Derm.Ab.
ug/l ug/l ug/cu m mg/kg mg/kg ug/kg ug/kg ug/kg ug/kg ug/kg mg/sq
cm
540-29-9 CHROMIUM (VI)
Soil
Dust/Soil Indoors 1.3e-008
Dust/Soil Outdoors 2.2e-008
NOTE: scientific notation is used for numbers less that 0.000001
and greater than 1000000.
For example:0.00000021 = 2.1e-7 = 2.1 / 10000000 and 21000000 =
2.1e7 = 2.1 * 10000000.
GW = Groundwater, SW = Surface Water, Sed = Sediment, Veget = Vegetable.
Derm.Ab. = Dermal Absorption Rate, (1) Indicates Outside Model Bounds,
(2) Indicates Missing Data
Exposure Parameters Used to Generate Exposure Estimates
The dose (or exposure concentration) values presented in this assessment reflect not only the concentrations of contaminants in various environmental media and the exposure pathways selected for analysis, but also the specific numerical parameters applied to each exposure scenario. The following tables summarize the exposure parameters used in this assessment.
Population: Avg American(RME)General Population Parameters
Body Weight: 70.00 kg
Lifetime: 70.00 years
Exposure Period: 30 years
Scenario Specific Parameters
Scenario General Parameters
Event Frequency Event Duration
Drinking Water 350 events per year
Indoor Air 350 events per year 21 hours per event
Outdoor Air 350 events per year 3 hours per event
Dust/Soil Indoors 350 events per year 21 hours per event
Dust/Soil Outdoors 350 events per year 3 hours per event
Scenario ORAL INHALATION DERMAL
Amount Fraction Breathing Exposed
Ingested Contamin. Rate Skin Area
Drinking Water 2 litersper event 100 %
Indoor Air 0.71 cu.m per hour
Outdoor Air 1.67 cu.m per hour
Dust/Soil Indoors 100 mg per event 100 % 0.71 cu.m per hour
Dust/Soil Outdoors 100 mg per event 100 % 1.67 cu.m per hour
Average Daily Dose or Exposure Concentration
When an exposure assessment will be used as part of a quantitative risk assessment, a numerical estimate of exposure must be calculated. The value employed for this estimate varies, according to the route of exposure.
When evaluating the risk of chronic non-cancer health effects from oral or dermal exposures, EPA employs the Average Daily Dose (ADD) received during the period of exposure. These are compared to Reference Doses (RfDs). When evaluating such effects from inhalation exposure, EPA employs contaminant concentrations, which are compared to Reference Concentrations (RfCs) for continuous exposure.
ADD =Average Daily Dose (during exposure period).
Units are milligrams of contaminant per kilogram of body weight
per day.
Inh.Conc =Concentration of contaminant in inhaled air.
Units are milligrams of contaminant per cubic meter of air.
Chemical Oral Inhalation Dermal
Medium ADD Concentration ADD
Scenario mg/kg/d mg/cu m mg/kg/d
18540-29-9 CHROMIUM (VI)
Groundwater
Drinking Water 0.000005
TOTALS 0.000005
Air
Indoor Air 0.000001
Outdoor Air 0.000001
TOTALS 0.000002
Soil
Dust/Soil Indoors 5.5e-007 1.3e-008
Dust/Soil Outdoors 5.5e-007 2.2e-008
TOTALS 0.000001 3.5e-008
NOTE: scientific notation is used for numbers less that 0.000001 and greater than 1000000. For example:0.00000021 = 2.1e-7 = 2.1 / 10000000 and 21000000 = 2.1e7 = 2.1 * 10000000. ADD/LADD values are meaningful up to the second significant digit.
Lifetime Average Daily Dose or Adjusted Exposure Concentration
When evaluating carcinogenic risks from exposures that last less than a lifetime, the ADD or exposure concentration is adjusted to a dose or concentration that would yield an equivalent exposure if exposure continued for the entire lifetime.
For oral or dermal exposures, this yields the Lifetime Average Daily Dose (LADD):
LADD = ADD * (exposure period in years / lifetime in years)
For inhalation exposures, this yields the Adjusted Concentration:
Adjusted Concentration = Concentration * (exposure period / lifetime)
Typically (and in RISK*ASSISTANT), the adjusted concentration will also incorporate adjustments for differences between the actual exposure pattern and the assumed pattern of continuous lifetime exposure. For example, if exposure only occurred for one hour each day, the Adjusted Concentration would be only 1/24th of the concentration during that hour.
LADD =Lifetime Average Daily Dose.
Units are milligrams of contaminant per kilogram of body weight per day.
Adj.Inh.Conc =Adjusted Inhaled Concentration: Continuous concentration
equivalent to
exposure concentration; considering frequency and duration of exposure
and inhalation rate.
Units are micrograms of contaminant per cubic meter.
Chemical Oral Inhalation Dermal
Medium LADD Adj.Concentration LADD
Scenario mg/kg/d ug/cu m mg/kg/d
18540-29-9 CHROMIUM (VI)
Groundwater
Drinking Water 0.000002
TOTALS 0.000002
Air
Indoor Air 0.000306
Outdoor Air 0.000103
TOTALS 0.000409
Soil
Dust/Soil Indoors 2.3e-007 0.000004
Dust/Soil Outdoors 2.3e-007 0.000002
TOTALS 4.7e-007 0.000006
NOTE: scientific notation is used for numbers less that 0.000001 and greater than 1000000. For example:0.00000021 = 2.1e-7 = 2.1 / 10000000 and 21000000 = 2.1e7 = 2.1 * 10000000. ADD/LADD values are meaningful up to the second significant digit.
Carcinogenic Risk
For chemicals that may cause cancer if ingested, risk is calculated as a function of oral Slope Factor and Dose:
-(Oral Slope Factor * Lifetime Average Daily Dose)
Risk = 1 - e
If the risk results from breathing the chemical, the calculation is based on concentration, rather than dose, as follows:
-(Unit Risk * Concentration)
Risk = 1 - e
For dermal exposures, the dose absorbed through the skin is used in combination with the oral slope factor, using the same equation that is used for calculating risks from oral exposures. This may lead to underestimation of dermal risk.
These estimates represent theoretical excess cancer risk (i.e. risk over background cancer incidence) of developing cancer. For example, if the calculated risk is 1 in 1,000,000 (1 e-006), this would literally suggest that a person would have a one-in-a-million chance of getting cancer because of the specified chemical exposure, in addition to her/his chance of getting cancer from other causes.
Oral Slope = Slope of the (carcinogenic) dose-response function,
in the low-dose, linear range.
Used for oral and dermal exposures.
Units are 1/(milligram of chemical per kilogram of body weight per
day).
Unit Risk = Slope of the (carcinogenic) concentration-response function,
in the low-concentration, linear range.
Used for inhalation exposures.
Units are 1/(micrograms of chemical per cubic meter of air).
Slope Factors and Unit Risks are generally estimated as the 95th percentile confidence limits using the linearized multistage model, when based on animal data (estimates derived from studies in humans often employ mathematical best estimates). As such, they are conservative estimates of toxic hazard. Risks estimated by combining these hazard values with exposure estimates are commonly referred to as upper-bound risks, but because exposure estimates may not represent upper-bound estimates, risk estimates are not true upper-bound risks.
Weight of Evidence = EPA class designating overall strength of
evidence that
a substance causes cancer in humans.
A = Known human carcinogen.
B1 = Probable human carcinogen, limited human data.
B2 = Probable human carcinogen, inadequate or no human data.
C = Possible human carcinogen.
D = Not classifiable as human carcinogen.
E = Evidence that not carcinogenic in humans.
Chemical Risk (Odds): Individual Probability of Getting Cancer
Medium from this Exposure Alone
Scenario Oral Inhalation Dermal
18540-29-9 CHROMIUM (VI)
Weight of Evidence: A No Slope Unit Risk(1/(ug/m3)):0.012 Source:
IRIS & HEAST(04/14/97&04/14/97)
Groundwater
Drinking Water Missing Slope
MEDIUM TOTALS
Air
Indoor Air 4 in 1,000,000 (4e-006)
Outdoor Air 1 in 1,000,000 (1e-006)
MEDIUM TOTALS 5 in 1,000,000 (5e-006)
Soil
Dust/Soil Indoors Missing Slope < 1 in 1,000,000 (5e-008)
Dust/Soil Outdoors Missing Slope < 1 in 1,000,000 (3e-008)
MEDIUM TOTALS < 1 in 1,000,000 (8e-008)
ALL MEDIA TOTALS 5 in 1,000,000 (5e-006)
NOTE: scientific notation is used for completeness. For example:0.00000021 = 2.1e-7 = 2.1 / 10,000,000 (odds of 2 in 10,000,000) and 21,000,000 = 2.1e7 = 2.1 * 10,000,000.
It is generally assumed that carcinogenic risk is zero only when exposure is zero, and that at low doses, the relationship between dose and response can be approximated by a straight line.
These estimates represent the theoretical excess cancer risk (i.e. risk over background cancer incidence) of developing cancer. For example, if the calculated risk is 0.000001 (1 e-006), this would literally suggest that a person would have a one-in-a-million chance of getting cancer because of the specified chemical exposure, in addition to her/his chance of getting cancer from other causes. However, in view of the large uncertainties associated with such risk estimates, they should always be interpreted as general indicators, rather than precise estimates. EPA generally considers risks below 1 in a 1,000,000 (1e-6) to be low.
Hazard Quotient
For agents that cause non-cancer toxic effects, a Hazard Quotient (H.Q.) is calculated, which compares the expected exposure to the agent to an exposure that is assumed not to be associated with toxic effects.
For oral or dermal exposures, the Average Daily Dose (ADD) is compared to a Reference Dose (RfD):
H.Q. = Average Daily Dose / Reference Dose
For inhalation exposures, the inhaled concentration is compared to a Reference Concentration (RfC):
H.Q. = Inhaled Concentration / Reference Concentration
An effort is made to ensure that Reference Doses and Reference Concentrations provide a conservative estimate of non-cancer toxic hazards. The uncertainty factors applied to toxicity data are intended to take into account differences in sensitivity to toxic effects within and between species, and differences in toxic effects between chronic and subchronic exposures.
Definitions of abbreviations employed in this table:
RfC =Reference Concentration (inhaled concentration not associated
with toxicity).
Units are milligrams of contaminant per cubic meter of air.
RfD =Reference Dose (daily dose not associated with toxicity).
Units are milligrams of contaminant per kilogram of body weight
per day.
Chemical Hazard Quotient: Ratio of Average Dose
Medium to 'Safe' Daily Dose
Scenario Oral Inhalation Dermal
18540-29-9 CHROMIUM (VI)
RfD (mg/kg/d): 0.005 No RfC Source: IRIS & HEAST(04/14/97&04/14/97)
Groundwater
Drinking Water 0.001096
MEDIUM TOTALS 0.001096
Air
Indoor Air Missing RfC
Outdoor Air Missing RfC
MEDIUM TOTALS
Soil
Dust/Soil Indoors 0.000110 Missing RfC
Dust/Soil Outdoors 0.000110 Missing RfC
ALL MEDIA TOTALS 0.001315
NOTE: scientific notation is used for completeness.
For example:0.00000021 = 2.1e-7 = 2.1 / 10,000,000 (odds of 2 in 10,000,000) and 21,000,000 = 2.1e7 = 2.1 * 10,000,000. HQ/HI values are meaningful up to the first significant digit.
It is generally assumed that non-cancer toxic effects have some threshold. That is, up to some finite level of exposure, physiological defense mechanisms ensure that no toxic effect will occur. Accordingly, hazard assessment for non-carcinogenic effects involve estimating an exposure that is less than this threshold level. This is done by applying "uncertainty factors" to exposures that appear to be near this threshold in laboratory toxicology studies. This yields a Reference Dose (RfD) for oral exposures, or a Reference Concentration (RfC) for inhalation exposures.
TOTALS FOR ALL CHEMICALS
Risk (Odds):Individual Probability of
Hazard Quotient Getting Cancer from this Exposure Alone
Oral 0.001315
Inhalation 5 in 1,000,000 (5e-006)
Groundwater 0.001096
Air 5 in 1,000,000 (5e-006)
Soil 0.000219
TOTAL 5 in 1,000,000 (5e-006)
NOTE: scientific notation is used for completeness.
For example:0.00000021 = 2.1e-7 = 2.1 / 10,000,000 (odds of 2 in 10,000,000) and 21,000,000 = 2.1e7 = 2.1 * 10,000,000. HQ/HI values are meaningful up to the first significant digit.
In some situations, it is appropriate for the user to calculate combined risks from multiple chemicals and multiple routes of exposure. Many chemicals will produce the same toxic effect, regardless of the exposure route. For chemicals that cause cancer by several routes of exposure, the combined risk from all routes may be more informative than route-specific risk estimates, unless there is evidence that carcinogenic risks from different routes reflect different mechanisms of action. Similarly, for non-cancer toxic effects, differences between routes may only affect toxic potency, which will be reflected in the use of route-specific Reference Doses or Reference Concentrations.
Carcinogenic risk estimates for particular chemicals and routes of exposure may be summed directly to produce an estimate of total carcinogenic risk. Similarly, Hazard Quotients for chemicals that produce toxic effects in the same organ system may be summed to yield a Hazard Index. Hazard Indices < 1.0 are generally considered by EPA to be associated with low risks on non-cancer toxic effects.
In generating estimates of the combined toxic and carcinogenic risks of different chemicals, it is also important to bear in mind that the risks of exposure to multiple chemicals are not necessarily additive. Risks may be less than additive, or synergism may lead to risks that are greater than would be predicted by an additive model. Unfortunately, only very limited data are available on the risks of exposure to multiple chemicals.
Carcinogenic risks that exceed 0.000001 (1 e-006), whether for a single chemical, route of exposure, and scenario, or for a combination of chemicals, exposure routes, and scenarios, fall within the EPA's range of concern. Depending upon the number of persons exposed to these risks and the plausibility of the assumptions underlying the estimate, some action to control the risks may be needed. Risks in excess of 0.0001 (1 e -004) are generally considered unacceptable.
In generating estimates of the combined toxic and carcinogenic risks of different chemicals, it is also important to bear in mind that the risks of exposure to multiple chemicals are not necessarily additive. Risks may be less than additive, or synergism may lead to risks that are greater than would be predicted by an additive model. Unfortunately, only very limited data are available on the risks of exposure to multiple chemicals.
Hazard Quotients and Hazard Indices that exceed 1.0, whether for a single chemical, route of exposure, and scenario, or for a combination of chemicals, exposure routes, and scenarios, indicate the possibility of non-cancer toxic risks from the exposure.
If a Hazard Index that exceeds 1.0 represents multiple chemicals and/or multiple routes of exposure, the assessor should ascertain that exposure to these chemicals/routes will lead to toxic effects in the same organ system. It may be appropriate to recalculate a Hazard Index that includes only those chemicals and routes of exposure that have overlapping patterns of toxicity.
