Basic Information
Note: EPA no longer updates this information, but it may be useful as a reference or resource.
From passenger vehicles to large transit bus fleets, fuel cells offer a promising new source of clean power for vehicles. This innovative technology uses chemical energy rather than combustion to generate electric power. It results in far fewer or even zero emissions.
Background
Fuel cells generate power through an electrochemical process, much like a battery. They convert chemical energy to electrical energy by combining hydrogen from fuel with oxygen from the air. Hydrogen fuel can be supplied in two ways - either directly as pure hydrogen gas or through a "fuel reformer" that converts hydrocarbon fuels such as methanol, natural gas, or gasoline into hydrogen-rich gas. (See Diagram.)
Performance
Because the technology is still under development, fuel cell vehicles' performance has not been well-studied or documented. Based on available research, fuel cell vehicles are expected to offer an extremely quiet ride with little vibration. Compared with conventional vehicles, fuel cell vehicles are also expected to provide improved fuel economy, increased engine efficiency, lower smog-forming emissions, and reduced greenhouse gas emissions. Fuel cells operating on pure hydrogen achieve zero emissions. Fuel cells can achieve 40 to 70 percent efficiency, which is substantially greater than the 30 percent efficiency of the most efficient internal combustion engines.
Diagram
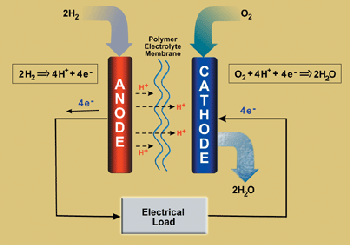
All fuel cells contain two electrodes - one positively and one negatively charged - with a substance that conducts electricity (electrolyte)sandwiched between them.
Availability
Most auto manufacturers are actively researching fuel cell transportation technologies and testing prototype passenger vehicles. Several North American cities are also testing fuel cell-powered transit buses.
Providing fuel to power these vehicles presents several challenges. Large investments are required to establish hydrogen production facilities and a convenient hydrogen distribution system to serve the general public. In the near term, pilot hydrogen fueling facilities are being developed that are based on liquid hydrogen, natural gas (steam methane reforming), and electricity (electrolysis). As an alternative, some manufacturers are considering using fuel reformers to allow fuel cell vehicles to use conventional fuels or chemical hydrogen storage.
Fuel Cell Types
All fuel cells contain two electrodes - one positively and one negatively charged - with a substance that conducts electricity (electrolyte) sandwiched between them. Fuel cells can achieve 40 to 70 percent efficiency, which is substantially greater than the 30 percent efficiency of the most efficient internal combustion engines. Differences in size, weight, cost and operating temperature all affect potential uses and for a variety of reasons, a number of fuel cell technologies are not practical for transportation. The Proton Exchange Membrane (PEM) fuel cell is the focus of vehicle-power research. The following are the major different types fuel cells:
- Proton Exchange Membrane (PEM -- sometimes also called "polymer electrolyte membrane") - Considered the leading fuel cell type for passenger car application; operates at relatively low temperatures and has a high power density.
- Phosphoric Acid - The most commercially developed fuel cell; generates electricity at more than 40 percent efficiency.
- Molten Carbonate - Promises high fuel-to-electricity efficiencies and the ability to utilize coal-based fuels.
- Solid Oxide - Can reach 60 percent power-generating efficiencies and be employed for large, high powered applications such as industrial generating stations.
- Alkaline - Used extensively by the space program; can achieve 70 percent power-generating efficiencies, but is considered too costly for transportation applications.
- Direct Methanol - Expected efficiencies of 40 percent with low operating temperatures; able to use hydrogen from methanol without a reformer. (A reformer is a device that produces hydrogen from another fuel like natural gas, methanol, or gasoline for use in a fuel cell.)
- Regenerative - Currently being researched by NASA; closed loop form of power generation that uses solar energy to separate water into hydrogen and oxygen.
Additional information about fuel cell technologies is available from the U.S. Department of Energy.
![[logo] US EPA](../gif/logo_epaseal.gif)