EPA On-line Tools for Site Assessment Calculation
| Module Home Objectives Table of Contents Previous < Next > |
| 13 of 45 |
Vapor Phase Partitioning
How about partitioning to the vapor phase? We know that MTBE is a volatile compound and even though it has one of the highest solubilities of the primary gasoline components, it still has a relatively low solubility in water. The Henry's Law constant of a compound defines the partitioning of that compound between the air and water phases.
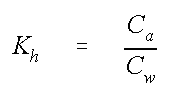
where Kh is the dimensionless henry's constant, Cw and Ca are the concentration in mass per volume units in water and air, respectively. The value of Kh will give us an indication if the compound "prefers" to stay in the water or in the air.
|
Home | Glossary | Notation | Links | References | Calculators |