Ecosystems Research
EPA On-line Tools for Site Assessment Calculation
| 37 of 67 |
Temperature Dependence of MTBE, Benzene and Toluene Solubility
Temperature dependence in effective solubility is introduced through data on methyl tert-butyl ether (MBTE) and benzene solubilities over the range of 0oC to 40 oC. These data are drawn from Peters et al., 20021 and Montegomery, 19962
Some comments are needed on these data:
- The temperature-dependent effective solubility calculator still uses the assumptions concerning Raoult's law as used in the original calculator:
- Mixture properties are approximated by the average properties of the fuel
- Unitary activity coefficients
- Inconsistent solubility data reported in the scientific literature is the rule rather than the exception. As new or improved data become available the calculator will be updated.
- The MTBE solubilities from Peters et al., 2002, do not match the commonly used value of about 50 g/L at 25oC. The Peters et al., data do, however, roughly match two data points reported by Fischer et al., 20043 , and the value reported given in the Handbook of Chemistry and Physics (Lide, 2000,4)
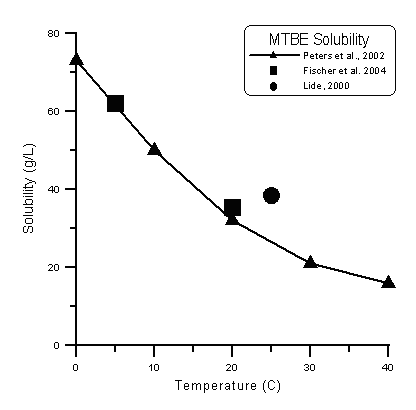
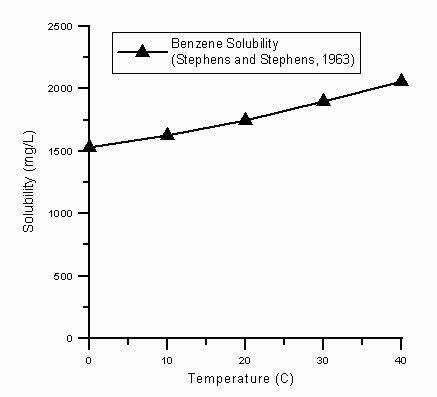
The calculator uses the data points from Peters et al. (triangles) and linear interpolation to estimate the MTBE solubility. Other data that would more fully establish the temperature-dependent solubility of MTBE do not exist. - The benzene data are taken from a larger list of contradictory data presented by Montegomery, but these (Stephens and Stephens, 1963)5 data were selected on the basis of their agreement with the reported solubility of benzene at 25oC.
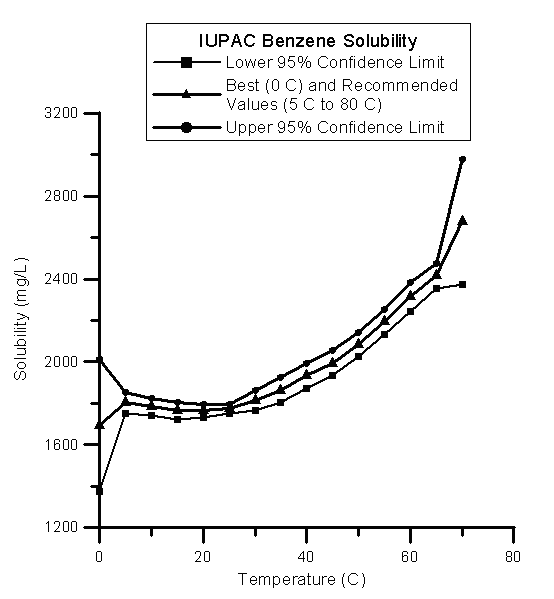
The International Union of Pure and Applied Chemistry (IUPAC)6 has evaluated benzene data from many sources and prepared a set of recommended values or best values for temperatures from 0oC to 80oC. Over the range of 0oC to 25oC the IUPAC data and its ranges encompasses the Stephens and Stephens (1963) data. IUPAC, however, shows that the solubility of Benzene remains roughly constant over this range. The data point for 0oC is a "best" value rather a "recommeded" value, because of more uncertainty at 0oC. The following table shows the values and ranges plotted in the following graph
| Temperature | Lower 95% Confidence limit | IUPAC Best or Recommended Values | Upper 95% Confidence limit |
|---|---|---|---|
| mg/L | mg/L | mg/L | |
| 0oC | 1372 | 1693 | 2013 |
| 5oC | 1753 | 1803 | 1853 |
| 10oC | 1743 | 1783 | 1823 |
| 15oC | 1723 | 1763 | 1803 |
| 20oC | 1733 | 1763 | 1793 |
| 25oC | 1753 | 1773 | 1793 |
Other sources of chemical data can be found on the ERD chemical properties page.
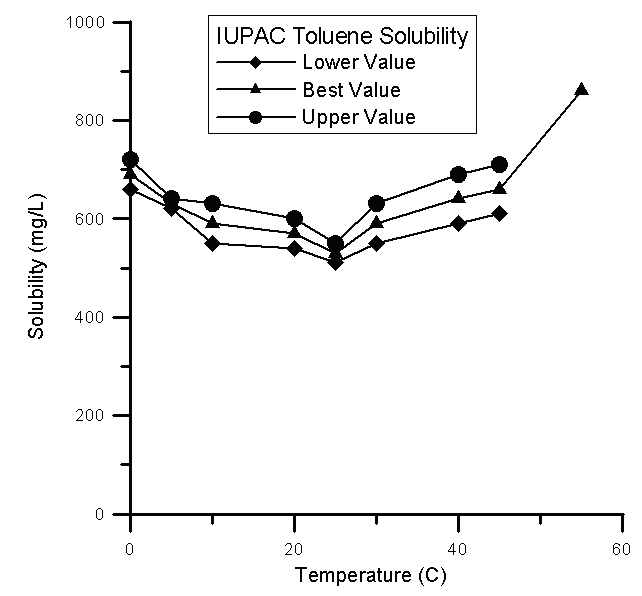
Toluene
The IUPAC prepared data on toluene6 and prepared a set of best values for temperatures from 0oC to 60oC. The following table shows the ranges of values for toluene and lower and upper ranges. These data were judged to be of generally lower quality and only "best" values rather than "recommended" values were given.
| Temperature | Lower 95% Confidence limit | IUPAC Best or Recommended Values | Upper 95% Confidence limit |
|---|---|---|---|
| mg/L | mg/L | mg/L | |
| 0oC | 660 | 690 | 720 |
| 5oC | 620 | 630 | 640 |
| 10oC | 550 | 590 | 630 |
| 20oC | 540 | 570 | 600 |
| 25oC | 510 | 530 | 550 |
References
1Peters, U., F. Nierlich, M. Sakuth, and M. Laugier, 2002, Methyl Tert-Butyl Ether, Physical and Chemical Properties, Ullmanns Encyclopedia of Industrial Chemistry, Release 2003, 6th ed., VCH Verlag GmbH & Co. KGaA, Wiley, DOI, 10.1002/14356007.a16_5432Montegomery, J.H., 1996, Groundwater Chemicals Desk Reference, 2nd ed., CRC Press, Lewis Publishers, Boca Raton, Florida, page 70-71.
3Fischer, A., M. Muller, J. Klasmeier, 2004, Determination of Henry's law constant for mthyl tert-butyl ether (MTBE) at groundwater temperatures, Chemosphere, 54, 689-694.
4Lide, D.R., 2000, CRC Handbook of Chemistry and Physics, 81st ed., CRC Press, 2000, page 8-95.
5Stephen, H. and T. Stephen, 1963, Solubilities of Inorganic and Organic Compounds, Vol. 1., Macmillan, New York.
6Shaw, D.G., 1989, Solubility Data Series, Hydrocarbon with Water and Seawater, Part I: Hydrocarbons C5 to C7, Volume 37, International Union of Pure and Applied Chemistry, Pergamon PRess, Oxford.
|
Top ^
Home | Glossary | Notation | Links | References | Calculators |