Chemical Safety for Sustainability (CSS)
Research at the Atlantic Ecology Division
Chemicals provide the key building blocks that are converted into end-use products or used in industrial processes to make products that create jobs and benefit society. Although chemicals are essential to modern life, we lack innovative, systematic, effective and efficient approaches and tools to inform decisions that reduce the environmental and societal impact of chemicals while increasing economic value. Improving the safe production, use and disposal of chemicals is a major priority of research at the U.S. Environmental Protection Agency (EPA) to support decisions and actions the Agency makes to meet its mission to protect human health and the environment. The Chemical Safety for Sustainability (CSS) program is primarily designed to assure the safety of chemicals and products that we use in our everyday lives and that impact the environment. CSS research provides decision-support tools needed to efficiently evaluate chemicals, conduct risk management, and prioritize time-critical research. AED is conducting research that will advance science to meet society’s current demands for a safer environment but to also meet the social, economic and environmental health needs of future generations.
Systems Models
AED researchers investigate the process of how a chemical interacts with human and wildlife biological processes. Innovative chemical screening technologies, such as automated, rapid screening will be used to generate chemical data on the biological effects of chemicals.
Project: Systems Approaches to Assess Human and Ecological Risks

US EPA regulations have been based traditionally upon the prevention of adverse biological effects of chemicals on individual organisms. However, environmental sustainability requires protection of the health of populations, communities and ecosystems. Thus, more comprehensive ecological risk assessment must link chemical sources to effects on aquatic/wildlife and ecological systems, and take into account the context in which chemical exposures occur, including physical stressors (e.g., habitat availability and quality) and biological factors (e.g., prey availability, predation). In this task, we will evaluate how integrated systems approaches can provide the framework, informed by mechanistic ecological and toxicological knowledge, for more efficient, comprehensive and realistic ecological risk assessments.
The Atlantic Ecology Division of the US EPA Office of Research and Development is contributing to this task in 3 areas:
- Molecular indicators for ecological risk assessment: Ecologically- and toxicologically-important genes are being discovered in common estuarine fish populations that may provide help predicting how human activities affect sensitive ecological species.
- Effects of multiple stressors on aquatic–dependent populations: Models describing population effects of environmental mercury exposure and habitat alteration are being developed and tested versus real –world data on wild loons of New Hampshire.
- Predicting chemical exposure and effects in Narragansett Bay. In coordination with the Narragansett Bay Signature Project that focuses on sustainable management of nitrogen, a case study is under development to provide information on the ecological risks of high priority watershed-wide residential/urban use chemicals such as pharmaceuticals and personal care products.
AED Task Lead: Diane Nacci
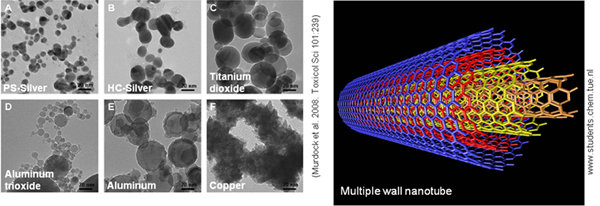
Project: An Integrated Systems Approach to Assess and Predict the Toxicity of Engineered Nanomaterials and their Applications
Research task: Systems-Based Approach for Assessing Hazard and Risk of Manufactured Nanomaterials and Non-Human Species and Ecosystems
This Task has three broad purposes:This task focuses on the ecotoxicology of manufactured nanomaterials (particles or fibers that have at least one dimension between 1 and 100 nm). These materials are of immediate concern to regulatory offices because they exhibit properties not observed in their traditional bulk form, are being developed and incorporated into products at a rapid rate, and require novel and non-standardized test approaches due to their particulate nature. In addition, due to their small size alone, these particles may be taken up and translocated via mechanisms very different from those typical of truly soluble substances. The inherent properties that determine toxicity might also differ significantly from traditional, soluble chemicals. These unique properties have the potential to elicit system-level responses at every level of organization, from the molecular/sub-cellular to the ecosystem (for example carbon or nutrient cycling).
- Develop methods for working with a broad range of nanomaterials to provide test guidance to regulatory Offices, primarily OCSPP.
- Characterize and quantify toxicity of various nanomaterials in marine systems to provide Offices with basic toxicity information but also to develop a basis for investigating chronic toxicity, mechanisms of action, toxic pathways, and initiating events, and ultimately to develop predictive tools to preclude extensive plant and animal testing.
- Develop methods and models to predict the hazard or ecological risk of nanomaterials.
AED Task Lead: Kay Ho
Extrapolation
AED researchers are investigating methods to accurately extrapolate exposure and effects of environmental contaminants from in vitro screening assays to in vivo human health and ecotoxicological impacts, as well as from one species to another (interspecies extrapolation). The focus is currently on endocrine-disrupting chemicals, specifically on endocrine active pharmaceuticals (EAPs).
Project: Extrapolation from In Vitro to In Vivo
Research task: In Vitro to In Vivo Exposure and Effects
Uncertainty regarding extrapolation of in vitro effects to an intact organism derives from the incomplete nature of in vitro assays with respect to the biochemical and physiological processes involved in the complex responses of a whole animal. Research is being conducted to determine the strengths and limitations of in vitro assays so that resulting data can be used to more accurately predict in vivo outcomes. Currently, this research is investigating the potential effects of endocrine-active pharmaceuticals (EAPs) on non-target species.
At AED, research is focused in four areas:
- laboratory experiments to determine how exposure to EAPs used in human hormone therapies affect reproductive output in an estuarine fish species (cunner, Tautogolabrus adspersus)
- the effects that EAPs have on the activity of a critical reproductive enzyme (aromatase) from fish exposed in vivo;
- an exploration of differences in metabolism of EAPs between species (e.g., fish, rats and humans), comparing results from in vivo and in vitro assays; and
- the development of methods for assessing the degree to which selective uptake of certain EAPs occurs through sex hormone binding globulin (SHBG) present in the gills of fish.
This research incorporates an integrated, trans-disciplinary approach by requiring the skills and expertise of analytical chemists, biologists, toxicologists, biochemists, and modelers. While the main goal of the research is to address the challenges associated with extrapolation, the results of this project will also provide information about whether management controls need to be changed or improved in order to prevent unintended exposure of non-target human populations, wildlife and fish to EAPs formulated for medical use.
AED Task Lead: Lesley Mills
![[logo] US EPA](../gif/logo_epaseal.gif)